Mycobacteria attenuate nociceptive responses by formyl peptide receptor triggered opioid peptide release from neutrophils
- PMID: 19343210
- PMCID: PMC2657213
- DOI: 10.1371/journal.ppat.1000362
Mycobacteria attenuate nociceptive responses by formyl peptide receptor triggered opioid peptide release from neutrophils
Abstract
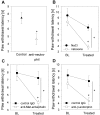
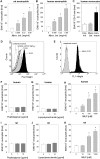
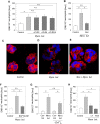
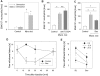
In inflammation, pain is regulated by a balance of pro- and analgesic mediators. Analgesic mediators include opioid peptides which are secreted by neutrophils at the site of inflammation, leading to activation of opioid receptors on peripheral sensory neurons. In humans, local opioids and opioid peptides significantly downregulate postoperative as well as arthritic pain. In rats, inflammatory pain is induced by intraplantar injection of heat inactivated Mycobacterium butyricum, a component of complete Freund's adjuvant. We hypothesized that mycobacterially derived formyl peptide receptor (FPR) and/or toll like receptor (TLR) agonists could activate neutrophils, leading to opioid peptide release and inhibition of inflammatory pain. In complete Freund's adjuvant-induced inflammation, thermal and mechanical nociceptive thresholds of the paw were quantified (Hargreaves and Randall-Selitto methods, respectively). Withdrawal time to heat was decreased following systemic neutrophil depletion as well as local injection of opioid receptor antagonists or anti-opioid peptide (i.e. Met-enkephalin, beta-endorphin) antibodies indicating an increase in pain. In vitro, opioid peptide release from human and rat neutrophils was measured by radioimmunoassay. Met-enkephalin release was triggered by Mycobacterium butyricum and formyl peptides but not by TLR-2 or TLR-4 agonists. Mycobacterium butyricum induced a rise in intracellular calcium as determined by FURA loading and calcium imaging. Opioid peptide release was blocked by intracellular calcium chelation as well as phosphoinositol-3-kinase inhibition. The FPR antagonists Boc-FLFLF and cyclosporine H reduced opioid peptide release in vitro and increased inflammatory pain in vivo while TLR 2/4 did not appear to be involved. In summary, mycobacteria activate FPR on neutrophils, resulting in tonic secretion of opioid peptides from neutrophils and in a decrease in inflammatory pain. Future therapeutic strategies may aim at selective FPR agonists to boost endogenous analgesia.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



Similar articles
-
Toll like receptor (TLR)-4 as a regulator of peripheral endogenous opioid-mediated analgesia in inflammation.Mol Pain. 2014 Feb 6;10:10. doi: 10.1186/1744-8069-10-10. Mol Pain. 2014. PMID: 24499354 Free PMC article.
-
Antinociception by neutrophil-derived opioid peptides in noninflamed tissue--role of hypertonicity and the perineurium.Brain Behav Immun. 2009 May;23(4):548-57. doi: 10.1016/j.bbi.2009.02.007. Epub 2009 Feb 20. Brain Behav Immun. 2009. PMID: 19233260
-
Recruitment of opioid peptide-containing neutrophils is independent of formyl peptide receptors.J Neuroimmunol. 2011 Jan;230(1-2):65-73. doi: 10.1016/j.jneuroim.2010.08.027. Epub 2010 Sep 25. J Neuroimmunol. 2011. PMID: 20869777
-
Endogenous opiates and behavior: 2012.Peptides. 2013 Dec;50:55-95. doi: 10.1016/j.peptides.2013.10.001. Epub 2013 Oct 12. Peptides. 2013. PMID: 24126281 Review.
-
Opioids in chronic pain.Eur J Pharmacol. 2001 Oct 19;429(1-3):79-91. doi: 10.1016/s0014-2999(01)01308-5. Eur J Pharmacol. 2001. PMID: 11698029 Review.
Cited by
-
The connection of monocytes and reactive oxygen species in pain.PLoS One. 2013 May 2;8(5):e63564. doi: 10.1371/journal.pone.0063564. Print 2013. PLoS One. 2013. PMID: 23658840 Free PMC article.
-
The Interplay between Chronic Pain, Opioids, and the Immune System.Neuroscientist. 2022 Dec;28(6):613-627. doi: 10.1177/10738584211030493. Epub 2021 Jul 16. Neuroscientist. 2022. PMID: 34269117 Free PMC article. Review.
-
De novo chemoattractants form supramolecular hydrogels for immunomodulating neutrophils in vivo.Bioconjug Chem. 2014 Dec 17;25(12):2116-22. doi: 10.1021/bc5004923. Epub 2014 Nov 25. Bioconjug Chem. 2014. PMID: 25398017 Free PMC article.
-
Distinct roles of exogenous opioid agonists and endogenous opioid peptides in the peripheral control of neuropathy-triggered heat pain.Sci Rep. 2016 Sep 8;6:32799. doi: 10.1038/srep32799. Sci Rep. 2016. PMID: 27605249 Free PMC article.
-
Neutrophil biology in injuries and diseases of the central and peripheral nervous systems.Prog Neurobiol. 2023 Sep;228:102488. doi: 10.1016/j.pneurobio.2023.102488. Epub 2023 Jun 23. Prog Neurobiol. 2023. PMID: 37355220 Free PMC article. Review.
References
-
- Xiao WH, Bennett GJ. C-fiber spontaneous discharge evoked by chronic inflammation is suppressed by a long-term infusion of lidocaine yielding nanogram per milliliter plasma levels. Pain. 2008;137:218–228. - PubMed
-
- Rittner HL, Machelska H, Stein C. Leukocytes in the regulation of pain and analgesia. J Leukoc Biol. 2005;79:1215–1222. - PubMed
-
- Tedesco LS, Fuseler J, Grisham M, Wolf R, Roerig SC. Therapeutic administration of nitric oxide synthase inhibitors reverses hyperalgesia but not inflammation in a rat model of polyarthritis. Pain. 2002;95:215–223. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

