Variable fitness impact of HIV-1 escape mutations to cytotoxic T lymphocyte (CTL) response
- PMID: 19343217
- PMCID: PMC2659432
- DOI: 10.1371/journal.ppat.1000365
Variable fitness impact of HIV-1 escape mutations to cytotoxic T lymphocyte (CTL) response
Abstract
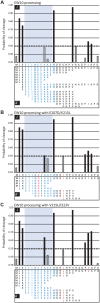
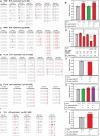
Human lymphocyte antigen (HLA)-restricted CD8(+) cytotoxic T lymphocytes (CTL) target and kill HIV-infected cells expressing cognate viral epitopes. This response selects for escape mutations within CTL epitopes that can diminish viral replication fitness. Here, we assess the fitness impact of escape mutations emerging in seven CTL epitopes in the gp120 Env and p24 Gag coding regions of an individual followed longitudinally from the time of acute HIV-1 infection, as well as some of these same epitopes recognized in other HIV-1-infected individuals. Nine dominant mutations appeared in five gp120 epitopes within the first year of infection, whereas all four mutations found in two p24 epitopes emerged after nearly two years of infection. These mutations were introduced individually into the autologous gene found in acute infection and then placed into a full-length, infectious viral genome. When competed against virus expressing the parental protein, fitness loss was observed with only one of the nine gp120 mutations, whereas four had no effect and three conferred a slight increase in fitness. In contrast, mutations conferring CTL escape in the p24 epitopes significantly decreased viral fitness. One particular escape mutation within a p24 epitope was associated with reduced peptide recognition and high viral fitness costs but was replaced by a fitness-neutral mutation. This mutation appeared to alter epitope processing concomitant with a reduced CTL response. In conclusion, CTL escape mutations in HIV-1 Gag p24 were associated with significant fitness costs, whereas most escape mutations in the Env gene were fitness neutral, suggesting a balance between immunologic escape and replicative fitness costs.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures





Similar articles
-
Fitness-Balanced Escape Determines Resolution of Dynamic Founder Virus Escape Processes in HIV-1 Infection.J Virol. 2015 Oct;89(20):10303-18. doi: 10.1128/JVI.01876-15. Epub 2015 Jul 29. J Virol. 2015. PMID: 26223634 Free PMC article.
-
Consequences of HLA-B*13-Associated Escape Mutations on HIV-1 Replication and Nef Function.J Virol. 2015 Nov;89(22):11557-71. doi: 10.1128/JVI.01955-15. Epub 2015 Sep 9. J Virol. 2015. PMID: 26355081 Free PMC article.
-
Effects of Mutations on Replicative Fitness and Major Histocompatibility Complex Class I Binding Affinity Are Among the Determinants Underlying Cytotoxic-T-Lymphocyte Escape of HIV-1 Gag Epitopes.mBio. 2017 Nov 28;8(6):e01050-17. doi: 10.1128/mBio.01050-17. mBio. 2017. PMID: 29184023 Free PMC article.
-
The influence of HLA/HIV genetics on the occurrence of elite controllers and a need for therapeutics geotargeting view.Braz J Infect Dis. 2021 Sep-Oct;25(5):101619. doi: 10.1016/j.bjid.2021.101619. Epub 2021 Sep 22. Braz J Infect Dis. 2021. PMID: 34562387 Free PMC article. Review.
-
Dynamic interplay between viral adaptation and immune recognition during HIV-1 infection.Protein Cell. 2010 Jun;1(6):514-9. doi: 10.1007/s13238-010-0068-0. Epub 2010 Jul 7. Protein Cell. 2010. PMID: 21204005 Free PMC article. Review.
Cited by
-
Balance between transmitted HLA preadapted and nonassociated polymorphisms is a major determinant of HIV-1 disease progression.J Exp Med. 2016 Sep 19;213(10):2049-63. doi: 10.1084/jem.20151984. Epub 2016 Aug 22. J Exp Med. 2016. PMID: 27551154 Free PMC article.
-
Fitness-Balanced Escape Determines Resolution of Dynamic Founder Virus Escape Processes in HIV-1 Infection.J Virol. 2015 Oct;89(20):10303-18. doi: 10.1128/JVI.01876-15. Epub 2015 Jul 29. J Virol. 2015. PMID: 26223634 Free PMC article.
-
Estimating the fitness cost of escape from HLA presentation in HIV-1 protease and reverse transcriptase.PLoS Comput Biol. 2012;8(5):e1002525. doi: 10.1371/journal.pcbi.1002525. Epub 2012 May 24. PLoS Comput Biol. 2012. PMID: 22654656 Free PMC article.
-
Partial escape of HIV-1 from cytotoxic T lymphocytes during chronic infection.J Virol. 2012 Jul;86(13):7459-63. doi: 10.1128/JVI.06724-11. Epub 2012 May 2. J Virol. 2012. PMID: 22553321 Free PMC article.
-
SARS-CoV-2 escape from cytotoxic T cells during long-term COVID-19.Nat Commun. 2023 Jan 10;14(1):149. doi: 10.1038/s41467-022-34033-x. Nat Commun. 2023. PMID: 36627290 Free PMC article.
References
-
- Goulder PJ, Watkins DI. HIV and SIV CTL escape: implications for vaccine design. Nat Rev Immunol. 2004;4:630–640. - PubMed
-
- Letvin NL. Progress toward an HIV vaccine. Annu Rev Med. 2005;56:213–223. - PubMed
-
- Allen TM, O'Connor DH, Jing P, Dzuris JL, Mothe BR, et al. Tat-specific cytotoxic T lymphocytes select for SIV escape variants during resolution of primary viraemia. Nature. 2000;407:386–390. - PubMed
-
- Borrow P, Lewicki H, Wei X, Horwitz MS, Peffer N, et al. Antiviral pressure exerted by HIV-1-specific cytotoxic T lymphocytes (CTLs) during primary infection demonstrated by rapid selection of CTL escape virus. Nat Med. 1997;3:205–211. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

