Myelination and regional domain differentiation of the axon
- PMID: 19343313
- PMCID: PMC2824168
- DOI: 10.1007/400_2009_3
Myelination and regional domain differentiation of the axon
Abstract
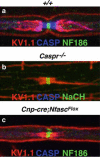
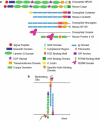
During evolution, as organisms increased in complexity and function, the need for the ensheathment and insulation of axons by glia became vital for faster conductance of action potentials in nerves. Myelination, as the process is termed, facilitates the formation of discrete domains within the axolemma that are enriched in ion channels, and macromolecular complexes consisting of cell adhesion molecules and cytoskeletal regulators. While it is known that glia play a substantial role in the coordination and organization of these domains, the mechanisms involved and signals transduced between the axon and glia, as well as the proteins regulating axo-glial junction formation remain elusive. Emerging evidence has shed light on the processes regulating myelination and domain differentiation, and key molecules have been identified that are required for their assembly and maintenance. This review highlights these recent findings, and relates their significance to domain disorganization as seen in several demyelinating disorders and other neuropathies.
Figures
Similar articles
-
Spatiotemporal ablation of myelinating glia-specific neurofascin (Nfasc NF155) in mice reveals gradual loss of paranodal axoglial junctions and concomitant disorganization of axonal domains.J Neurosci Res. 2009 Jun;87(8):1773-93. doi: 10.1002/jnr.22015. J Neurosci Res. 2009. PMID: 19185024 Free PMC article.
-
Nodes of Ranvier in health and disease.J Peripher Nerv Syst. 2023 Jul;28 Suppl 3:S3-S11. doi: 10.1111/jns.12568. J Peripher Nerv Syst. 2023. PMID: 37272548 Review.
-
Gangliosides contribute to stability of paranodal junctions and ion channel clusters in myelinated nerve fibers.Glia. 2007 May;55(7):746-57. doi: 10.1002/glia.20503. Glia. 2007. PMID: 17352383
-
Organization and maintenance of molecular domains in myelinated axons.J Neurosci Res. 2013 May;91(5):603-22. doi: 10.1002/jnr.23197. Epub 2013 Feb 13. J Neurosci Res. 2013. PMID: 23404451 Free PMC article. Review.
-
Rapid disruption of axon-glial integrity in response to mild cerebral hypoperfusion.J Neurosci. 2011 Dec 7;31(49):18185-94. doi: 10.1523/JNEUROSCI.4936-11.2011. J Neurosci. 2011. PMID: 22159130 Free PMC article.
Cited by
-
Axonal ensheathment and intercellular barrier formation in Drosophila.Int Rev Cell Mol Biol. 2010;283:93-128. doi: 10.1016/S1937-6448(10)83003-5. Int Rev Cell Mol Biol. 2010. PMID: 20801419 Free PMC article. Review.
-
A Novel Mutation in CNTNAP1 Gene Causes Disorganization of Axonal Domains, Hypomyelination and Severe Neurological Deficits.J Neurosci Res. 2025 Apr;103(4):e70040. doi: 10.1002/jnr.70040. J Neurosci Res. 2025. PMID: 40265789
-
Elovl5 is required for proper action potential conduction along peripheral myelinated fibers.Glia. 2021 Oct;69(10):2419-2428. doi: 10.1002/glia.24048. Epub 2021 Jun 17. Glia. 2021. PMID: 34139039 Free PMC article.
-
A versatile genetic tool to study midline glia function in the Drosophila CNS.Dev Biol. 2017 Sep 1;429(1):35-43. doi: 10.1016/j.ydbio.2017.06.010. Epub 2017 Jun 9. Dev Biol. 2017. PMID: 28602954 Free PMC article.
-
Postnatal Loss of Neuronal and Glial Neurofascins Differentially Affects Node of Ranvier Maintenance and Myelinated Axon Function.Front Cell Neurosci. 2017 Feb 3;11:11. doi: 10.3389/fncel.2017.00011. eCollection 2017. Front Cell Neurosci. 2017. PMID: 28217083 Free PMC article.
References
-
- Abe I, Ochiai N, Ichimura H, Tsujino A, Sun J, Hara Y. Internodes can nearly double in length with gradual elongation of the adult rat sciatic nerve. J Orthop Res. 2004;22:571–577. - PubMed
-
- Arroyo EJ, Xu YT, Zhou L, Messing A, Peles E, Chiu SY, Scherer SS. Myelinating Schwann cells determine the internodal localization of Kv1.1, Kv1.2, Kvbeta2, and Caspr. J Neurocytol. 1999;28:333–347. - PubMed
-
- Baba H, Akita H, Ishibashi T, Inoue Y, Nakahira K, Ikenaka K. Completion of myelin compaction, but not the attachment of oligodendroglial processes triggers K+ channel clustering. J Neurosci Res. 1999;58:752–764. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

