Axonal protein synthesis and the regulation of local mitochondrial function
- PMID: 19343315
- PMCID: PMC2786086
- DOI: 10.1007/400_2009_1
Axonal protein synthesis and the regulation of local mitochondrial function
Abstract
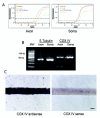
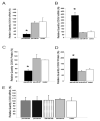
Axons and presynaptic nerve terminals of both invertebrate and mammalian SCG neurons contain a heterogeneous population of nuclear-encoded mitochondrial mRNAs and a local cytosolic protein synthetic system. Nearly one quarter of the total protein synthesized in these structural/functional domains of the neuron is destined for mitochondria. Acute inhibition of axonal protein synthesis markedly reduces the functional activity of mitochondria. The blockade of axonal protein into mitochondria had similar effects on the organelle's functional activity. In addition to mitochondrial mRNAs, SCG axons contain approximately 200 different microRNAs (miRs), short, noncoding RNA molecules involved in the posttranscriptional regulation of gene expression. One of these miRs (miR-338) targets cytochrome c oxidase IV (COXIV) mRNA. This nuclear-encoded mRNA codes for a protein that plays a key role in the assembly of the mitochondrial enzyme complex IV and oxidative phosphorylation. Over-expression of miR-338 in the axon markedly decreases COXIV expression, mitochondrial functional activity, and the uptake of neurotransmitter into the axon. Conversely, the inhibition of endogeneous miR-338 levels in the axon significantly increased mitochondrial activity and norepinephrine uptake into the axon. The silencing of COXIV expression in the axon using short, inhibitory RNAs (siRNAs) yielded similar results, a finding that indicated that the effects of miR-338 on mitochondrial activity and axon function were mediated, at least in part, through local COXIV mRNA translation. Taken together, recent findings establish that proteins requisite for mitochondrial activity are synthesized locally in the axon and nerve terminal, and call attention to the intimacy of the relationship that has evolved between the distant cellular domains of the neuron and its energy generating systems.
Figures






Similar articles
-
MicroRNA-338 regulates the axonal expression of multiple nuclear-encoded mitochondrial mRNAs encoding subunits of the oxidative phosphorylation machinery.Cell Mol Life Sci. 2012 Dec;69(23):4017-27. doi: 10.1007/s00018-012-1064-8. Epub 2012 Jul 8. Cell Mol Life Sci. 2012. PMID: 22773120 Free PMC article.
-
Dysregulation of the axonal trafficking of nuclear-encoded mitochondrial mRNA alters neuronal mitochondrial activity and mouse behavior.Dev Neurobiol. 2014 Mar;74(3):333-50. doi: 10.1002/dneu.22141. Epub 2013 Nov 20. Dev Neurobiol. 2014. PMID: 24151253 Free PMC article.
-
MicroRNA-338 regulates local cytochrome c oxidase IV mRNA levels and oxidative phosphorylation in the axons of sympathetic neurons.J Neurosci. 2008 Nov 19;28(47):12581-90. doi: 10.1523/JNEUROSCI.3338-08.2008. J Neurosci. 2008. PMID: 19020050 Free PMC article.
-
Nuclear-Encoded Mitochondrial mRNAs: A Powerful Force in Axonal Growth and Development.Neuroscientist. 2018 Apr;24(2):142-155. doi: 10.1177/1073858417714225. Epub 2017 Jun 14. Neuroscientist. 2018. PMID: 28614981 Review.
-
Local mRNA translation in long-term maintenance of axon health and function.Curr Opin Neurobiol. 2020 Aug;63:15-22. doi: 10.1016/j.conb.2020.01.006. Epub 2020 Feb 19. Curr Opin Neurobiol. 2020. PMID: 32087477 Review.
Cited by
-
MicroRNA-338 regulates the axonal expression of multiple nuclear-encoded mitochondrial mRNAs encoding subunits of the oxidative phosphorylation machinery.Cell Mol Life Sci. 2012 Dec;69(23):4017-27. doi: 10.1007/s00018-012-1064-8. Epub 2012 Jul 8. Cell Mol Life Sci. 2012. PMID: 22773120 Free PMC article.
-
MicroRNAs in the axon and presynaptic nerve terminal.Front Cell Neurosci. 2013 Aug 6;7:126. doi: 10.3389/fncel.2013.00126. eCollection 2013. Front Cell Neurosci. 2013. PMID: 23964201 Free PMC article.
-
Regulation of axonal trafficking of cytochrome c oxidase IV mRNA.Mol Cell Neurosci. 2010 Apr;43(4):422-30. doi: 10.1016/j.mcn.2010.01.009. Epub 2010 Feb 6. Mol Cell Neurosci. 2010. PMID: 20144716 Free PMC article.
-
Dysregulation of the axonal trafficking of nuclear-encoded mitochondrial mRNA alters neuronal mitochondrial activity and mouse behavior.Dev Neurobiol. 2014 Mar;74(3):333-50. doi: 10.1002/dneu.22141. Epub 2013 Nov 20. Dev Neurobiol. 2014. PMID: 24151253 Free PMC article.
-
Late Endosomes Act as mRNA Translation Platforms and Sustain Mitochondria in Axons.Cell. 2019 Jan 10;176(1-2):56-72.e15. doi: 10.1016/j.cell.2018.11.030. Epub 2019 Jan 3. Cell. 2019. PMID: 30612743 Free PMC article.
References
-
- Bauer MF, Hofman S. Import of mitochondrial proteins. In: Shapira AHV, editor. Mitochondrial function and dysfunction. Academic Press; San Diego, CA: 2006. pp. 57–90.
-
- Beaumont V, Zhong N, Fletcher R, Froemke RC, Zucker RS. Phosphorylation and local presynaptic protein synthesis in calcium- and calcineurin-dependent induction of crayfish long-term facilitation. Neuron. 2001;32:489–501. - PubMed
-
- Bleher R, Martin R. Ribosomes in the squid giant axon. Neuroscience. 2001;107:527–534. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

