High-throughput capillary-electrophoresis analysis of the contents of a single mitochondria
- PMID: 19344146
- PMCID: PMC2682631
- DOI: 10.1021/ac900099y
High-throughput capillary-electrophoresis analysis of the contents of a single mitochondria
Abstract
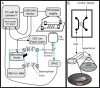
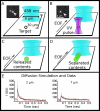
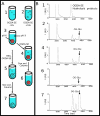
We present a technique for labeling the contents of acidic organelles and rapidly releasing, separating, and detecting their labeled contents with laser-induced fluorescence. We have performed solution-phase separation of the contents of single mitochondria and single 100 nm vesicles, which represents a demonstration of an analyzed volume of approximately 1 aL. Our strategy to label the acidic contents of the mitochondrion relies on the use of the membrane-permeable dye, Oregon Green diacetate succinimidyl ester, and a membrane-permeable base to raise intramitochondrial pH. In order to measure the contents, we utilized a glass microfluidic chip and high voltage gradient for millisecond capillary electrophoresis separation after single-mitochondrion photolysis. We observed heterogeneity among a population of mitochondria with respect to a constituent chemical component.
Figures


Similar articles
-
[Studies on single-cell analysis].Se Pu. 2007 Jan;25(1):1-10. Se Pu. 2007. PMID: 17432566 Review. Chinese.
-
Microfluidic chip for plasma separation from undiluted human whole blood samples using low voltage contactless dielectrophoresis and capillary force.Lab Chip. 2014 Jun 21;14(12):1996-2001. doi: 10.1039/c4lc00196f. Epub 2014 May 12. Lab Chip. 2014. PMID: 24817130
-
Microfluidic picoliter-scale translational spontaneous sample introduction for high-speed capillary electrophoresis.Anal Chem. 2009 May 1;81(9):3693-8. doi: 10.1021/ac900573x. Anal Chem. 2009. PMID: 19351143
-
High-throughput multiplexed capillary electrophoresis in drug discovery.Drug Discov Today. 2004 Dec 15;9(24):1072-80. doi: 10.1016/S1359-6446(04)03293-3. Drug Discov Today. 2004. PMID: 15582796 Review.
-
High performance microfluidic capillary electrophoresis devices.Biomed Microdevices. 2007 Jun;9(3):405-12. doi: 10.1007/s10544-007-9049-3. Biomed Microdevices. 2007. PMID: 17487587
Cited by
-
Single-cell nanosurgery.Methods Mol Biol. 2013;991:139-48. doi: 10.1007/978-1-62703-336-7_14. Methods Mol Biol. 2013. PMID: 23546666 Free PMC article.
-
Micro- and Nano-Devices for Studying Subcellular Biology.Small. 2021 Jan;17(3):e2005793. doi: 10.1002/smll.202005793. Epub 2020 Dec 20. Small. 2021. PMID: 33345457 Free PMC article. Review.
-
A hyphenated optical trap capillary electrophoresis laser induced native fluorescence system for single-cell chemical analysis.Analyst. 2012 Jul 7;137(13):2965-72. doi: 10.1039/c2an35198f. Epub 2012 Apr 30. Analyst. 2012. PMID: 22543409 Free PMC article.
-
Controlling mass transport in microfluidic devices.Annu Rev Anal Chem (Palo Alto Calif). 2011;4:275-96. doi: 10.1146/annurev-anchem-061010-113926. Annu Rev Anal Chem (Palo Alto Calif). 2011. PMID: 21456968 Free PMC article. Review.
-
Microsecond analysis of transient molecules using bi-directional capillary electrophoresis.Anal Chem. 2009 Nov 1;81(21):8790-6. doi: 10.1021/ac901283y. Anal Chem. 2009. PMID: 19874052 Free PMC article.
References
-
- Duffy CF, MacCraith B, Diamond D, O'Kennedy R, Arriaga EA. Lab Chip. 2006;6:1007–1011. - PubMed
-
- Fuller KM, Arriaga EA. Curr. Opin. Biotech. 2003;14:35–41. - PubMed
-
- Fuller KM, Duffy CF, Arriaga EA. Electrophoresis. 2002;23:1571–1576. - PubMed
-
- Strack A, Duffy C. n. F., Malvey M, Arriaga EA. Analytical Biochemistry. 2001;294:141–147. - PubMed
-
- Zischka H, Larochette N, Hoffmann F, Hamoller D, Jagemann N, Lichtmannegger J, Jennen L, Muller-Hocker J, Roggel F, Gottlicher M, Vollmar AM, Kroemer G. Anal. Chem. 2008;80:5051–5058. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

