Proton-coupled electron transfer in biology: results from synergistic studies in natural and model systems
- PMID: 19344235
- PMCID: PMC4625787
- DOI: 10.1146/annurev.biochem.78.080207.092132
Proton-coupled electron transfer in biology: results from synergistic studies in natural and model systems
Abstract
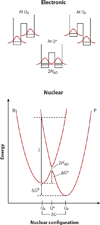
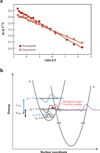
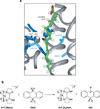
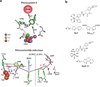
Proton-coupled electron transfer (PCET) underpins energy conversion in biology. PCET may occur with the unidirectional or bidirectional transfer of a proton and electron and may proceed synchronously or asynchronously. To illustrate the role of PCET in biology, this review presents complementary biological and model systems that explore PCET in electron transfer (ET) through hydrogen bonds [azurin as compared to donor-acceptor (D-A) hydrogen-bonded networks], the activation of C-H bonds [alcohol dehydrogenase and soybean lipoxygenase (SLO) as compared to Fe(III) metal complexes], and the generation and transport of amino acid radicals [photosystem II (PSII) and ribonucleotide reductase (RNR) as compared to tyrosine-modified photoactive Re(I) and Ru(II) complexes]. In providing these comparisons, the fundamental principles of PCET in biology are illustrated in a tangible way.
Figures







References
-
- Cukier RI, Nocera DG. Proton-coupled electron transfer. Annu. Rev. Phys. Chem. 1998;49:337–369. - PubMed
-
- Mayer JM. Proton-coupled electron transfer: a reaction chemist’s view. Annu. Rev. Phys. Chem. 2004;55:363–390. - PubMed
-
- Pourbaix M. Atlas of Electrochemical Equilibria in Aqueous Solutions. Oxford: Pergamon; 1966.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

