Collagen structure and stability
- PMID: 19344236
- PMCID: PMC2846778
- DOI: 10.1146/annurev.biochem.77.032207.120833
Collagen structure and stability
Abstract
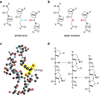
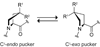
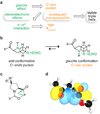
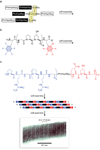
Collagen is the most abundant protein in animals. This fibrous, structural protein comprises a right-handed bundle of three parallel, left-handed polyproline II-type helices. Much progress has been made in elucidating the structure of collagen triple helices and the physicochemical basis for their stability. New evidence demonstrates that stereoelectronic effects and preorganization play a key role in that stability. The fibrillar structure of type I collagen-the prototypical collagen fibril-has been revealed in detail. Artificial collagen fibrils that display some properties of natural collagen fibrils are now accessible using chemical synthesis and self-assembly. A rapidly emerging understanding of the mechanical and structural properties of native collagen fibrils will guide further development of artificial collagenous materials for biomedicine and nanotechnology.
Figures



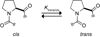


References
LITERATURE CITED
-
- Brinckmann J. Collagens at a glance. Top. Curr. Chem. 2005;247:1–-6.
-
- Veit G, Kobbe B, Keene DR, Paulsson M, Koch M, Wagener R. Collagen XXVIII, a novel von Willebrand factor A domain-containing protein with many imperfections in the collagenous domain. J. Biol. Chem. 2006;281:3494–3504. - PubMed
-
- Schweitzer MH, Suo Z, Avci R, Asara JM, Allen MA, et al. Analyses of soft tissue from Tyrannosaurus rex suggest the presence of protein. Science. 2007;316:277–280. - PubMed
-
- Asara JM, Schweitzer MH, Freimark LM, Phillips M, Cantley LC. Protein sequences from mastodon and Tyrannosaurus rex revealed by mass spectrometry. Science. 2007;316:280–285. - PubMed
RELATED RESOURCES
-
- Dalgleish R. A database of osteogenesis imperfecta and type III collagen mutations. 2009. http://www.le.ac.uk/genetics/collagen/
-
- Khoshnoodi J, Cartailler J-P, Alvares K, Veis A, Hudson BG. Computer-generated animation of assembly of type I and type IV collagen for Reference 38. 2006. http://www.mc.vanderbilt.edu/cmb/collagen/
-
- Ricard-Blum S, Ruggiero F, van der Rest M. The collagen superfamily. Top. Curr. Chem. 2005;247:35–84.
-
- Koide T, Nagata K. Collagen biosynthesis. Top. Curr. Chem. 2005;247:85–114.
-
- Greenspan DS. Biosynthetic processing of collagen molecules. Top. Curr. Chem. 2005;247:149–183.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

