Axonal mitochondrial clusters containing mutant SOD1 in transgenic models of ALS
- PMID: 19344250
- PMCID: PMC2842590
- DOI: 10.1089/ars.2009.2614
Axonal mitochondrial clusters containing mutant SOD1 in transgenic models of ALS
Abstract
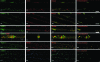
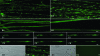
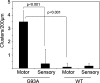
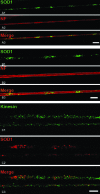
We studied the subcellular distribution of mitochondria and superoxide dismutase-1 (SOD1) in whole mounts of microdissected motor axons of rats expressing the ALS-linked SOD1-G93A mutation. The rationale was to determine whether physical interactions between the enzyme and mitochondria were linked to the axonopathy of motor fibers occurring in amyotrophic lateral sclerosis (ALS). Mitochondria and SOD1 displayed a homogeneous distribution along motor axons both in nontransgenic rats and in those overexpressing wild-type SOD1. In contrast, axons from SOD1-G93A rats (older than 35 days) showed accumulation of mitochondria in discrete clusters located at regular intervals. Most of SOD1 immunoreactivity was enriched in these clusters and colocalized with mitochondria, suggesting a recruitment of SOD1-G93A to the organelle. The SOD1/mitochondrial clusters were abundant in motor axons but scarcely seen in sensory axons. Clusters also were stained for neuronal nitric oxide synthase, nitrotyrosine, and cytochrome c. The later also was detected surrounding clusters. Ubiquitin colocalized with clusters only at late stages of the disease. The cytoskeleton was not overtly altered in clusters. These results suggest that mutant SOD1 and defective mitochondria create localized dysfunctional domains in motor axons, which may lead to progressive axonopathy in ALS.
Figures





References
-
- Abe K. Pan LH. Watanabe M. Kato T. Itoyama Y. Induction of nitrotyrosine-like immunoreactivity in the lower motor neuron of amyotrophic lateral sclerosis. Neurosci Lett. 1995;199:152–154. - PubMed
-
- Alvarez B. Demicheli V. Duran R. Trujillo M. Cervenansky C. Freeman BA. Radi R. Inactivation of human Cu,Zn superoxide dismutase by peroxynitrite and formation of histidinyl radical. Free Radic Biol Med. 2004;37:813–822. - PubMed
-
- Andersen PM. Genetics of sporadic ALS. Amyotroph Lateral Scler Other Motor Neuron Disord. 2001;2(suppl 1):S37–S41. - PubMed
-
- Basso M. Massignan T. Samengo G. Cheroni C. De Biasi S. Salmona M. Bendotti C. Bonetto V. Insoluble mutant SOD1 is partly oligoubiquitinated in amyotrophic lateral sclerosis mice. J Biol Chem. 2006;281:33325–33335. - PubMed
-
- Beckman JS. Estevez AG. Crow JP. Barbeito L. Superoxide dismutase and the death of motoneurons in ALS. Trends Neurosci. 2001;24:S15–S20. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

