Design and application of oncolytic HSV vectors for glioblastoma therapy
- PMID: 19344302
- PMCID: PMC3219506
- DOI: 10.1586/ern.09.9
Design and application of oncolytic HSV vectors for glioblastoma therapy
Abstract
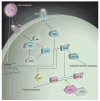
Glioblastoma multiforme is one of the most common human brain tumors. The tumor is generally highly infiltrative, making it extremely difficult to treat by surgical resection or radiotherapy. This feature contributes to recurrence and a very poor prognosis. Few anticancer drugs have been shown to alter rapid tumor growth and none are ultimately effective. Oncolytic vectors have been employed as a treatment alternative based on the ability to tailor virus replication to tumor cells. The human neurotropic herpes simplex virus (HSV) is especially attractive for development of oncolytic vectors (oHSV) because this virus is highly infectious, replicates rapidly and can be readily modified to achieve vector attenuation in normal brain tissue. Tumor specificity can be achieved by deleting viral genes that are only required for virus replication in normal cells and permit mutant virus replication selectively in tumor cells. The anti-tumor activity of oHSV can be enhanced by arming the vector with genes that either activate chemotherapeutic drugs within the tumor tissue or promote anti-tumor immunity. In this review, we describe current designs of oHSV and the experience thus far with their potential utility for glioblastoma therapy. In addition, we discuss the impediments to vector effectiveness and describe our view of future developments in vector improvement.
Figures
References
-
- McLendon RE, Halperin EC. Is the long-term survival of patients with intracranial glioblastoma multiforme overstated? Cancer. 2003;98(8):1745–1748. - PubMed
-
- Davis FG, Freels S, Grutsch J, Barlas S, Brem S. Survival rates in patients with primary malignant brain tumors stratified by patient age and tumor histological type: an analysis based on Surveillance, Epidemiology, and End Results (SEER) data, 1973-1991. J Neurosurg. 1998;88(1):1–10. - PubMed
-
- Trouillas P, Menaud G, De Thé G, Aimard G, Devic M. Epidemiological study of primary tumors of the neuraxis in the Rhone-Alps region. Quantitative data on the etiology and geographical distribution of 1670 tumors. Rev Neurol (Paris) 1975;131(10):691–708. - PubMed
-
- Soffer D, Gomori JM, Pomeranz S, Siegal T. Gliomas following low-dose irradiation to the head report of three cases. J Neurooncol. 1990;8(1):67–72. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
