Preferential priming of alloreactive T cells with indirect reactivity
- PMID: 19344462
- PMCID: PMC5990255
- DOI: 10.1111/j.1600-6143.2009.02578.x
Preferential priming of alloreactive T cells with indirect reactivity
Abstract
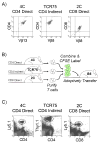
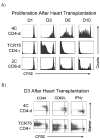
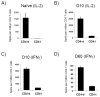
The relative contributions of the direct and indirect pathways in alloimmune responses have not been fully elucidated. We report a novel murine TCR transgenic system that can simultaneously track the CD4-direct (CD4-d), CD4-indirect (CD4-i) and CD8-direct (CD8-d) pathways after transplantation. Using this system, we have observed a profoundly greater proliferation of CD4-i T cells relative to CD4-d and CD8-d T cells after transplantation. Furthermore, a much larger proportion of CD4-i T cells attain an effector phenotype. We also analyzed endogenous, wild-type T cells using enzyme-linked immunospot analysis. In naïve mice, T cells with indirect reactivity were undetectable, but T cells with direct reactivity were abundant. However, 10 days after skin or heterotopic heart transplantation, CD4-i T cells comprised approximately 10% of the CD4+ response. Consistent with increased priming of the CD4-i pathway, we observed that the CD4-i T cells were further enriched in the effector cells migrating to the allograft and in memory-like T cells persisting after rejection. Thus, priming of the CD4-i pathway is favored after transplantation, allowing a rare population to rapidly become a major component of the CD4+ T-cell response in acute allograft rejection. The generalizability of this observation to other models remains to be determined.
Figures





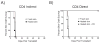
Comment in
-
Direct versus indirect allorecognition pathways: on the right track.Am J Transplant. 2009 Apr;9(4):655-6. doi: 10.1111/j.1600-6143.2009.02572.x. Am J Transplant. 2009. PMID: 19344457 Free PMC article. No abstract available.
References
-
- Rogers NJ, Lechler RI. Allorecognition. Am J Transplant. 2001 Jul;1(2):97–102. - PubMed
-
- Suchin EJ, Langmuir PB, Palmer E, Sayegh MH, Wells AD, Turka LA. Quantifying the frequency of alloreactive T cells in vivo: new answers to an old question. J Immunol. 2001 Jan 15;166(2):973–81. - PubMed
-
- Baker RJ, Hernandez-Fuentes MP, Brookes PA, Chaudhry AN, Cook HT, Lechler RI. Loss of direct and maintenance of indirect alloresponses in renal allograft recipients: implications for the pathogenesis of chronic allograft nephropathy. J Immunol. 2001 Dec 15;167(12):7199–206. - PubMed
-
- Vella JP, Vos L, Carpenter CB, Sayegh MH. Role of indirect allorecognition in experimental late acute rejection. Transplantation. 1997 Dec 27;64(12):1823–8. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

