Modulations of benzo[a]pyrene-induced DNA adduct, cyclin D1 and PCNA in oral tissue by 1,4-phenylenebis(methylene)selenocyanate
- PMID: 19344691
- PMCID: PMC2693912
- DOI: 10.1016/j.bbrc.2009.03.145
Modulations of benzo[a]pyrene-induced DNA adduct, cyclin D1 and PCNA in oral tissue by 1,4-phenylenebis(methylene)selenocyanate
Abstract
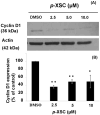
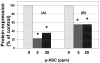
Tobacco smoking is an important cause of human oral squamous cell carcinoma (SCC). Tobacco smoke contains multiple carcinogens include polycyclic aromatic hydrocarbons typified by benzo[a]pyrene (B[a]P). Surgery is the conventional treatment approach for SCC, but it remains imperfect. However, chemoprevention is a plausible strategy and we had previously demonstrated that 1,4-phenylenebis(methylene)selenocyanate (p-XSC) significantly inhibited tongue tumors-induced by the synthetic 4-nitroquinoline-N-oxide (not present in tobacco smoke). In this study, we demonstrated that p-XSC is capable of inhibiting B[a]P-DNA adduct formation, cell proliferation, cyclin D1 expression in human oral cells in vitro. In addition, we showed that dietary p-XSC inhibits B[a]P-DNA adduct formation, cell proliferation and cyclin D1 protein expression in the mouse tongue in vivo. The results of this study are encouraging to further evaluate the chemopreventive efficacy of p-XSC initially against B[a]P-induced tongue tumors in mice and ultimately in the clinic.
Figures


Similar articles
-
Chemoprevention of lung tumorigenesis induced by a mixture of benzo(a)pyrene and 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone by the organoselenium compound 1,4-phenylenebis(methylene)selenocyanate.Cancer Lett. 2000 Dec 8;161(1):35-46. doi: 10.1016/s0304-3835(00)00590-5. Cancer Lett. 2000. PMID: 11078911
-
Comparative action of 1,4-phenylenebis(methylene)selenocyanate and its metabolites against 7,12-dimethylbenz[a]anthracene-DNA adduct formation in the rat and cell proliferation in rat mammary tumor cells.Chem Biol Interact. 2003 Oct 25;146(2):179-90. doi: 10.1016/j.cbi.2003.08.004. Chem Biol Interact. 2003. PMID: 14597131
-
Effects of dietary 1,4-phenylenebis(methylene)selenocyanate on 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone-induced DNA adduct formation in lung and liver of A/J mice and F344 rats.Carcinogenesis. 1996 Apr;17(4):749-53. doi: 10.1093/carcin/17.4.749. Carcinogenesis. 1996. PMID: 8625486
-
Chemoprevention of cancer by organoselenium compounds.J Cell Biochem Suppl. 1995;22:92-100. doi: 10.1002/jcb.240590812. J Cell Biochem Suppl. 1995. PMID: 8538214 Review.
-
Mechanisms of mammary cancer chemoprevention by organoselenium compounds.Mutat Res. 2004 Jul 13;551(1-2):181-97. doi: 10.1016/j.mrfmmm.2004.02.023. Mutat Res. 2004. PMID: 15225592 Review.
Cited by
-
Black Raspberry Extract Enhances Glutathione Conjugation of the Fjord-Region Diol Epoxide Derived from the Tobacco Carcinogen Dibenzo[def,p]chrysene in Human Oral Cells.Chem Res Toxicol. 2022 Nov 21;35(11):2152-2159. doi: 10.1021/acs.chemrestox.2c00246. Epub 2022 Oct 19. Chem Res Toxicol. 2022. PMID: 36260657 Free PMC article.
-
Melanoma prevention using topical PBISe.Cancer Prev Res (Phila). 2011 Jun;4(6):935-48. doi: 10.1158/1940-6207.CAPR-10-0202. Epub 2011 Mar 2. Cancer Prev Res (Phila). 2011. PMID: 21367959 Free PMC article.
-
Will chronic e-cigarette use cause lung disease?Am J Physiol Lung Cell Mol Physiol. 2015 Dec 15;309(12):L1398-409. doi: 10.1152/ajplung.00272.2015. Epub 2015 Sep 25. Am J Physiol Lung Cell Mol Physiol. 2015. PMID: 26408554 Free PMC article. Review.
-
Syringic acid may attenuate the oral mucosal carcinogenesis via improving cell surface glycoconjugation and modifying cytokeratin expression.Toxicol Rep. 2018 Oct 28;5:1098-1106. doi: 10.1016/j.toxrep.2018.10.015. eCollection 2018. Toxicol Rep. 2018. PMID: 30425931 Free PMC article.
-
To Cut the Mustard: Antimicrobial Activity of Selenocyanates on the Plate and in the Gas Phase.Antibiotics (Basel). 2023 Feb 1;12(2):290. doi: 10.3390/antibiotics12020290. Antibiotics (Basel). 2023. PMID: 36830201 Free PMC article.
References
-
- Canto MT, Devesa SS. Oral cavity and pharynx cancer incidence rates in the United States, 1975-1998. Oral Oncol. 2002;38:610–7. - PubMed
-
- Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, Thun MJ. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96. - PubMed
-
- Magrath I, Litvak J. Cancer in developing countries: opportunity and challenge. J Natl Cancer Inst. 1993;85:862–74. - PubMed
-
- Parkin DM, Pisani P, Ferlay J. Global cancer statistics. CA Cancer J Clin. 1999;49:33–64. 1. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

