Genome instability, cancer and aging
- PMID: 19344750
- PMCID: PMC4354930
- DOI: 10.1016/j.bbagen.2009.03.020
Genome instability, cancer and aging
Abstract
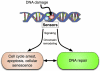
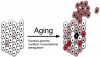
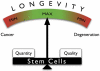
DNA damage-driven genome instability underlies the diversity of life forms generated by the evolutionary process but is detrimental to the somatic cells of individual organisms. The cellular response to DNA damage can be roughly divided in two parts. First, when damage is severe, programmed cell death may occur or, alternatively, temporary or permanent cell cycle arrest. This protects against cancer but can have negative effects on the long term, e.g., by depleting stem cell reservoirs. Second, damage can be repaired through one or more of the many sophisticated genome maintenance pathways. However, erroneous DNA repair and incomplete restoration of chromatin after damage is resolved, produce mutations and epimutations, respectively, both of which have been shown to accumulate with age. An increased burden of mutations and/or epimutations in aged tissues increases cancer risk and adversely affects gene transcriptional regulation, leading to progressive decline in organ function. Cellular degeneration and uncontrolled cell proliferation are both major hallmarks of aging. Despite the fact that one seems to exclude the other, they both may be driven by a common mechanism. Here, we review age-related changes in the mammalian genome and their possible functional consequences, with special emphasis on genome instability in stem/progenitor cells.
Figures

References
-
- de Duve C. The onset of selection. Nature. 2005;433:581–582. - PubMed
-
- Friedberg EC, Walker GR, Siede W, Wood RD, Schultz RA, Ellenberger T. DNA Repair and Mutagenesis. 2nd ASM Press; Washington: 2006.
-
- Kirkwood TB. Evolution of ageing. Nature. 1977;270:301–304. - PubMed
-
- Vijg J. Aging of the Genome. Oxford University Press; New York: 2007.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

