Molecular dynamics simulations of membrane channels and transporters
- PMID: 19345092
- PMCID: PMC2680122
- DOI: 10.1016/j.sbi.2009.02.011
Molecular dynamics simulations of membrane channels and transporters
Abstract
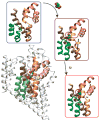
Membrane transport constitutes one of the most fundamental processes in all living cells with proteins as major players. Proteins as channels provide highly selective diffusive pathways gated by environmental factors, and as transporters furnish directed, energetically uphill transport consuming energy. X-ray crystallography of channels and transporters furnishes a rapidly growing number of atomic resolution structures, permitting molecular dynamics (MD) simulations to reveal the physical mechanisms underlying channel and transporter function. Ever increasing computational power today permits simulations stretching up to 1 micros, that is, to physiologically relevant time scales. Membrane protein simulations presently focus on ion channels, on aquaporins, on protein-conducting channels, as well as on various transporters. In this review we summarize recent developments in this rapidly evolving field.
Figures
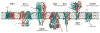

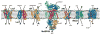
References
-
- Lindahl E, Sansom MSP. Membrane proteins: molecular dynamics simulations. Curr Opin Struct Biol. 2008;18:425–431. - PubMed
-
- Hashido M, Kidera A, Ikeguchi M. Water transport in aquaporins: osmotic permeability matrix analysis of molecular dynamics simulations. Biophys J. 2007;93:373–385. Osmotic permeability of water in aquaporins is described by a matrix that can be decomposed into contributions from each local region of the channel. - PMC - PubMed
-
- Zhu F, Tajkhorshid E, Schulten K. Collective diffusion model for water permeation through microscopic channels. Phys Rev Lett. 2004;93:224501. - PubMed
-
- Chen H, Ilan B, Wu Y, Zhu F, Schulten K, Voth GA. Charge delocalization in proton channels. I. The aquaporin channels and proton blockage. Biophys J. 2007;92:46–60. Mechanism of proton blockage in aquaporins is investigated by a combination of steered MD simulations and quantum chemistry calculations. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

