Merlin and the ERM proteins--regulators of receptor distribution and signaling at the cell cortex
- PMID: 19345106
- PMCID: PMC2796113
- DOI: 10.1016/j.tcb.2009.02.006
Merlin and the ERM proteins--regulators of receptor distribution and signaling at the cell cortex
Abstract
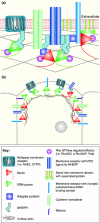
Recent studies highlight the importance of the distribution of membrane receptors in controlling receptor output and in contributing to complex biological processes. The cortical cytoskeleton is known to affect membrane protein distribution but the molecular basis of this is largely unknown. Here, we discuss the functions of Merlin and the ERM proteins both in linking membrane proteins to the underlying cortical cytoskeleton and in controlling the distribution of and signaling from membrane receptors. We also propose a model that could account for the intricacies of Merlin function across model organisms.
Figures

References
-
- Popowicz GM, et al. Filamins: promiscuous organizers of the cytoskeleton. Trends Biochem. Sci. 2006;31:411–419. - PubMed
-
- Bennett V, Baines AJ. Spectrin and ankyrin-based pathways: metazoan inventions for integrating cells into tissues. Physiol. Rev. 2001;81:1353–1392. - PubMed
-
- Sheetz MP, et al. Continuous membrane-cytoskeleton adhesion requires continuous accommodation to lipid and cytoskeleton dynamics. Annu. Rev. Biophys. Biomol. Struct. 2006;35:417–434. - PubMed
-
- Sheetz MP. Cell control by membrane-cytoskeleton adhesion. Nat. Rev. Mol. Cell Biol. 2001;2:392–396. - PubMed
-
- Kaksonen M, et al. Harnessing actin dynamics for clathrin-mediated endocytosis. Nat. Rev. Mol. Cell Biol. 2006;7:404–414. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

