Molecular and functional characterization of adipokinetic hormone receptor and its peptide ligands in Bombyx mori
- PMID: 19345219
- PMCID: PMC2745557
- DOI: 10.1016/j.febslet.2009.03.060
Molecular and functional characterization of adipokinetic hormone receptor and its peptide ligands in Bombyx mori
Abstract
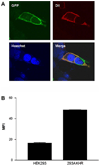
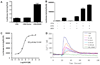
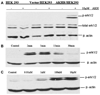
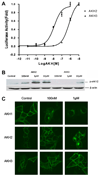
Neuropeptides of the adipokinetic hormone (AKH) family are among the best studied hormone peptides, but its signaling pathways remain to be elucidated. In this study, we molecularly characterized the signaling of Bombyx AKH receptor (AKHR) and its peptide ligands in HEK293 cells. In HEK293 cells stably expressing AKHR, AKH1 stimulation not only led to a ligand concentration dependent mobilization of intracellular Ca(2+) and cAMP accumulation, but also elicited transient activation of extracellular signal-regulated kinase 1/2 (ERK1/2) pathway. We observed that AKH receptor was rapidly internalized after AKH1 stimulation. We further demonstrated that AKH2 exhibited high activities in cAMP accumulation and ERK1/2 activation on AKHR comparable to AKH1, whereas AKH3 was much less effective.
Figures

References
-
- Schooneveld H, Tesser GI, Veenstra JA, Romberg-Privee HM. Adipokinetic hormone and AKH-like peptide demonstrated in the corpora cardiaca and nervous system of Locusta migratoria by immunocytochemistry. Cell Tissue Res. 1983;230:67–76. - PubMed
-
- Oudejans RC, Kooiman FP, Heerma W, Versluis C, Slotboom AJ, Beenakkers MT. Isolation and structure elucidation of a novel adipokinetic hormone (Lom-AKH-III) from the glandular lobes of the corpus cardiacum of the migratory locust, Locusta migratoria. Eur. J. Biochem. 1991;195:351–359. - PubMed
-
- Gade G, Janssens MP, Kellner R. A novel peptide in the AKH/RPCH family isolated from the corpora cardiaca of the Emperor dragonfly, Anax imperator. Peptides. 1994;15:1–6. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

