Mitochondrial free calcium regulation during sarcoplasmic reticulum calcium release in rat cardiac myocytes
- PMID: 19345225
- PMCID: PMC2683203
- DOI: 10.1016/j.yjmcc.2009.03.015
Mitochondrial free calcium regulation during sarcoplasmic reticulum calcium release in rat cardiac myocytes
Abstract
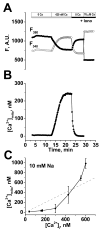
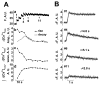
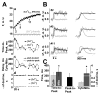
Cardiac mitochondria can take up Ca(2+), competing with Ca(2+) transporters like the sarcoplasmic reticulum (SR) Ca(2+)-ATPase. Rapid mitochondrial [Ca(2+)] transients have been reported to be synchronized with normal cytosolic [Ca(2+)](i) transients. However, most intra-mitochondrial free [Ca(2+)] ([Ca(2+)](mito)) measurements have been uncalibrated, and potentially contaminated by non-mitochondrial signals. Here we measured calibrated [Ca(2+)](mito) in single rat myocytes using the ratiometric Ca(2+) indicator fura-2 AM and plasmalemmal permeabilization by saponin (to eliminate cytosolic fura-2). The steady-state [Ca(2+)](mito) dependence on [Ca(2+)](i) (with 5 mM EGTA) was sigmoid with [Ca(2+)](mito)<[Ca(2+)](i) for [Ca(2+)](i) below 475 nM. With low [EGTA] (50 microM) and 150 nM [Ca(2+)](i) (+/-15 mM Na(+)) cyclical spontaneous SR Ca(2+) release occurred (5-15/min). Changes in [Ca(2+)](mito) during individual [Ca(2+)](i) transients were small ( approximately 2-10 nM/beat), but integrated gradually to steady-state. Inhibition SR Ca(2+) handling by thapsigargin, 2 mM tetracaine or 10 mM caffeine all stopped the progressive rise in [Ca(2+)](mito) and spontaneous Ca(2+) transients (confirming that SR Ca(2+) releases caused the [Ca(2+)](mito) rise). Confocal imaging of local [Ca(2+)](mito) (using rhod-2) showed that [Ca(2+)](mito) rose rapidly with a delay after SR Ca(2+) release (with amplitude up to 10 nM), but declined much more slowly than [Ca(2+)](i) (time constant 2.8+/-0.7 s vs. 0.19+/-0.06 s). Total Ca(2+) uptake for larger [Ca(2+)](mito) transients was approximately 0.5 micromol/L cytosol (assuming 100:1 mitochondrial Ca(2+) buffering), consistent with prior indirect estimates from [Ca(2+)](i) measurements, and corresponds to approximately 1% of the SR Ca(2+) uptake during a normal Ca(2+) transient. Thus small phasic [Ca(2+)](mito) transients and gradually integrating [Ca(2+)](mito) signals occur during repeating [Ca(2+)](i) transients.
Figures





Comment in
-
Ca2+ dynamics in the mitochondria - state of the art.J Mol Cell Cardiol. 2011 Nov;51(5):627-31. doi: 10.1016/j.yjmcc.2011.08.003. Epub 2011 Aug 16. J Mol Cell Cardiol. 2011. PMID: 21864537 Free PMC article.
References
-
- Denton RM, McCormack JG. Ca2+ as a second messenger within mitochondria of the heart and other tissues. Annu Rev Physiol. 1990;52:451–66. - PubMed
-
- Bers DM. Calcium cycling and signaling in cardiac myocytes. Annu Rev Physiol. 2008;70:23–49. - PubMed
-
- Gunter TE, Pfeiffer DR. Mechanisms by which mitochondria transport calcium. Am J Physiol. 1990 May;258(5 Pt 1):C755–86. - PubMed
-
- Kirichok Y, Krapivinsky G, Clapham DE. The mitochondrial calcium uniporter is a highly selective ion channel. Nature. 2004 Jan 22;427(6972):360–4. - PubMed
-
- Buntinas L, Gunter KK, Sparagna GC, Gunter TE. The rapid mode of calcium uptake into heart mitochondria (RaM): comparison to RaM in liver mitochondria. Biochim Biophys Acta. 2001 Apr 2;1504(2–3):248–61. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

