Rapid 3-D imaging of contrast flow: application in a perfused kidney phantom
- PMID: 19345468
- PMCID: PMC3134266
- DOI: 10.1016/j.ultrasmedbio.2008.10.016
Rapid 3-D imaging of contrast flow: application in a perfused kidney phantom
Abstract
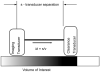
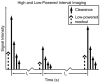
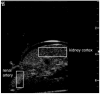
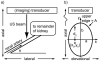
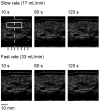
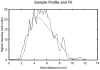
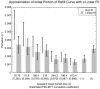
Previous studies indicate imaging of ultrasound contrast in 3-D is potentially superior to 2-D imaging for vascular characterization. A dual-beam, dynamic refill technique, which relies on volumetric contrast clearance and sequential imaging, was used to image a preserved porcine kidney perfused with contrast. A model was developed for the contrast profile across the renal artery to estimate fractional blood volume. This model was used along with refill curve information to measure absolute perfusion within renal cortex for a 100-cm(3) volume. Perfusion measurements from a slice within the volume were also made using a modified interval imaging technique. The measured perfusion using the dual-beam technique was consistent with the perfusion measured using the interval imaging technique (dual-beam values were 1.06 +/- 0.04 x corresponding interval imaging values). These experiments suggest that ultrasound contrast perfusion measurements are independent of the volume of contrast eliminated before refill.
Figures










References
-
- Arditi M, Frinking PJA, Zhou X, Rognin NG. A New Formalism for the Quantification of Tissue Perfusion by the Destruction-Replenishment Method in Contrast Ultrasound Imaging. IEEE Trans Ultrason Ferroelect, Freq Contr. 2006;53(6):1118–1129. - PubMed
-
- Billingsley P. Probability and Measure. Second Edition. New York: John Wiley & Sons, Inc.; 1986.
-
- Bhatti PT, LeCarpentier GL, Roubidoux MA, Fowlkes JB, Helvie MA, Carson PL. Discrimination of Sonographic Breast Lesions Using Frequency Shift Color Doppler Imaging, Age and Gray Scale Criteria. J Ultrasound Med. 2001;20:343–350. - PubMed
-
- Carson PL, Moskalik AP, Govil A, Roubidoux MA, Fowlkes JB, Normolle D, Adler DD, Rubin JM, Helvie M. The 3D and 2D Color Flow Display of Breast Masses. Ultrasound Med Biol. 1997;23(6):837–849. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

