Transport of mannose-6-phosphate receptors from the trans-Golgi network to endosomes requires Rab31
- PMID: 19345684
- PMCID: PMC2753287
- DOI: 10.1016/j.yexcr.2009.03.020
Transport of mannose-6-phosphate receptors from the trans-Golgi network to endosomes requires Rab31
Abstract
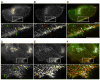
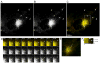
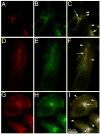
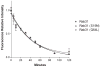
Rab31, a protein that we originally cloned from a rat oligodendrocyte cDNA library, localizes in the trans-Golgi network (TGN) and endosomes. However, its function has not yet been established. Here we show the involvement of Rab31 in the transport of mannose 6-phosphate receptors (MPRs) from TGN to endosomes. We demonstrate the specific sorting of cation-dependent-MPR (CD-MPR), but not CD63 and vesicular stomatitis virus G (VSVG) protein, to Rab31-containing trans-Golgi network carriers. CD-MPR and Rab31 containing carriers originate from extending TGN tubules that also contain clathrin and GGA1 coats. Expression of constitutively active Rab31 reduced the content of CD-MPR in the TGN relative to that of endosomes, while expression of dominant negative Rab31 triggered reciprocal changes in CD-MPR distribution. Expression of dominant negative Rab31 also inhibited the formation of carriers containing CD-MPR in the TGN, without affecting the exit of VSVG from this compartment. Importantly, siRNA-mediated depletion of endogenous Rab31 caused the collapse of the Golgi apparatus. Our observations demonstrate that Rab31 is required for transport of MPRs from TGN to endosomes and for the Golgi/TGN organization.
Figures





Similar articles
-
Interaction of Rab31 and OCRL-1 in oligodendrocytes: its role in transport of mannose 6-phosphate receptors.J Neurosci Res. 2010 Feb 15;88(3):589-604. doi: 10.1002/jnr.22236. J Neurosci Res. 2010. PMID: 19795375 Free PMC article.
-
Dynamics of Rab7b-dependent transport of sorting receptors.Traffic. 2012 Sep;13(9):1273-85. doi: 10.1111/j.1600-0854.2012.01388.x. Epub 2012 Jul 17. Traffic. 2012. PMID: 22708738
-
Endosome to Golgi transport of ricin is independent of clathrin and of the Rab9- and Rab11-GTPases.Mol Biol Cell. 2001 Jul;12(7):2099-107. doi: 10.1091/mbc.12.7.2099. Mol Biol Cell. 2001. PMID: 11452006 Free PMC article.
-
Visualization of TGN-endosome trafficking in mammalian and Drosophila cells.Methods Enzymol. 2012;504:255-71. doi: 10.1016/B978-0-12-391857-4.00013-6. Methods Enzymol. 2012. PMID: 22264539 Review.
-
Receptor-mediated sorting of soluble vacuolar proteins: myths, facts, and a new model.J Exp Bot. 2016 Aug;67(15):4435-49. doi: 10.1093/jxb/erw222. Epub 2016 Jun 4. J Exp Bot. 2016. PMID: 27262127 Review.
Cited by
-
Peptides targeting RAB11A-FIP2 complex inhibit HPIV3, RSV, and IAV replication as broad-spectrum antivirals.Cell Biosci. 2025 Apr 21;15(1):50. doi: 10.1186/s13578-025-01384-z. Cell Biosci. 2025. PMID: 40259361 Free PMC article.
-
Insulin-Like Growth Factor 2 As a Possible Neuroprotective Agent and Memory Enhancer-Its Comparative Expression, Processing and Signaling in Mammalian CNS.Int J Mol Sci. 2021 Feb 12;22(4):1849. doi: 10.3390/ijms22041849. Int J Mol Sci. 2021. PMID: 33673334 Free PMC article. Review.
-
Therapeutic Targeting of Rab GTPases: Relevance for Alzheimer's Disease.Biomedicines. 2022 May 16;10(5):1141. doi: 10.3390/biomedicines10051141. Biomedicines. 2022. PMID: 35625878 Free PMC article. Review.
-
Rab GTPases: Emerging Oncogenes and Tumor Suppressive Regulators for the Editing of Survival Pathways in Cancer.Cancers (Basel). 2020 Jan 21;12(2):259. doi: 10.3390/cancers12020259. Cancers (Basel). 2020. PMID: 31973201 Free PMC article. Review.
-
RAB31 Targeted by MiR-30c-2-3p Regulates the GLI1 Signaling Pathway, Affecting Gastric Cancer Cell Proliferation and Apoptosis.Front Oncol. 2018 Nov 26;8:554. doi: 10.3389/fonc.2018.00554. eCollection 2018. Front Oncol. 2018. PMID: 30534536 Free PMC article.
References
-
- Rohn WM, Rouille Y, Waguri S, Hoflack B. Bi-directional trafficking between the trans-Golgi network and the endosomal/lysosomal system. J Cell Sci. 2000;113 ( Pt 12):2093–2101. - PubMed
-
- Rothman JE. Mechanisms of intracellular protein transport. Nature. 1994;372:55–63. - PubMed
-
- Bonifacino JS, Glick BS. The mechanisms of vesicle budding and fusion. Cell. 2004;116:153–166. - PubMed
-
- Armstrong J. How do Rab proteins function in membrane traffic? Int J Biochem Cell Biol. 2000;32:303–307. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

