A stem cell niche for intermediate progenitor cells of the embryonic cortex
- PMID: 19346271
- PMCID: PMC2693531
- DOI: 10.1093/cercor/bhp029
A stem cell niche for intermediate progenitor cells of the embryonic cortex
Abstract
The excitatory neurons of the mammalian cerebral cortex arise from asymmetric divisions of radial glial cells in the ventricular zone and symmetric division of intermediate progenitor cells (IPCs) in the subventricular zone (SVZ) of the embryonic cortex. Little is known about the microenvironment in which IPCs divide or whether a stem cell niche exists in the SVZ of the embryonic cortex. Recent evidence suggests that vasculature may provide a niche for adult stem cells but its role in development is less clear. We have investigated the vasculature in the embryonic cortex during neurogenesis and find that IPCs are spatially and temporally associated with blood vessels during cortical development. Intermediate progenitors mimic the pattern of capillaries suggesting patterns of angiogenesis and neurogenesis are coordinated during development. More importantly, we find that IPCs divide near blood vessel branch points suggesting that cerebral vasculature establishes a stem cell niche for intermediate progenitors in the SVZ. These data provide novel evidence for the presence of a neurogenic niche for intermediate progenitors in the embryonic SVZ and suggest blood vessels are important for proper patterning of neurogenesis.
Figures
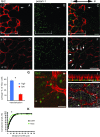
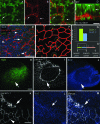
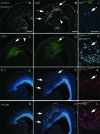
References
-
- Anthony TE, Klein C, Fishell G, Heintz N. Radial glia serve as neuronal progenitors in all regions of the central nervous system. Neuron. 2004;41:881–890. - PubMed
-
- Breier G, Albrecht U, Sterrer S, Risau W. Expression of vascular endothelial growth factor during embryonic angiogenesis and endothelial cell differentiation. Development. 1992;114:521–532. - PubMed
-
- Breier G, Clauss M, Risau W. Coordinate expression of vascular endothelial growth factor receptor-1 (flt-1) and its ligand suggests a paracrine regulation of murine vascular development. Dev Dyn. 1995;204:228–239. - PubMed
-
- Calabrese C, Poppleton H, Kocak M, Hogg TL, Fuller C, Hamner B, Oh EY, Gaber MW, Finklestein D, Allen M, et al. A perivascular niche for brain tumor stem cells. Cancer Cell. 2007;11:69–82. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

