Methodological problems in the use of indirect comparisons for evaluating healthcare interventions: survey of published systematic reviews
- PMID: 19346285
- PMCID: PMC2665205
- DOI: 10.1136/bmj.b1147
Methodological problems in the use of indirect comparisons for evaluating healthcare interventions: survey of published systematic reviews
Abstract
Objective: To investigate basic assumptions and other methodological problems in the application of indirect comparison in systematic reviews of competing healthcare interventions.
Design: Survey of published systematic reviews. Inclusion criteria Systematic reviews published between 2000 and 2007 in which an indirect approach had been explicitly used.
Data extraction: Identified reviews were assessed for comprehensiveness of the literature search, method for indirect comparison, and whether assumptions about similarity and consistency were explicitly mentioned.
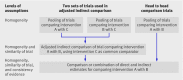
Results: The survey included 88 review reports. In 13 reviews, indirect comparison was informal. Results from different trials were naively compared without using a common control in six reviews. Adjusted indirect comparison was usually done using classic frequentist methods (n=49) or more complex methods (n=18). The key assumption of trial similarity was explicitly mentioned in only 40 of the 88 reviews. The consistency assumption was not explicit in most cases where direct and indirect evidence were compared or combined (18/30). Evidence from head to head comparison trials was not systematically searched for or not included in nine cases.
Conclusions: Identified methodological problems were an unclear understanding of underlying assumptions, inappropriate search and selection of relevant trials, use of inappropriate or flawed methods, lack of objective and validated methods to assess or improve trial similarity, and inadequate comparison or inappropriate combination of direct and indirect evidence. Adequate understanding of basic assumptions underlying indirect and mixed treatment comparison is crucial to resolve these methodological problems. APPENDIX 1: PubMed search strategy. APPENDIX 2: Characteristics of identified reports. APPENDIX 3: Identified studies. References of included studies.
Conflict of interest statement
Competing interests: None declared.
Figures
References
-
- Pocok SJ. Clinical trials: a practical approach. New York: Wiley, 1996.
-
- Glenny AM, Altman DG, Song F, Sakarovitch C, Deeks JJ, D’Amico R, et al. Indirect comparisons of competing interventions. Health Technol Assess 2005;9:1-134. - PubMed
-
- Ioannidis JP. Indirect comparisons: the mesh and mess of clinical trials. Lancet 2006;368:1470-2. - PubMed
-
- Bucher HC, Guyatt GH, Griffith LE, Walter SD. The results of direct and indirect treatment comparisons in meta-analysis of randomized controlled trials. J Clin Epidemiol 1997;50:683-91. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical