Organoselenium coating on cellulose inhibits the formation of biofilms by Pseudomonas aeruginosa and Staphylococcus aureus
- PMID: 19346348
- PMCID: PMC2687307
- DOI: 10.1128/AEM.02683-08
Organoselenium coating on cellulose inhibits the formation of biofilms by Pseudomonas aeruginosa and Staphylococcus aureus
Abstract
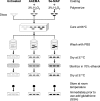
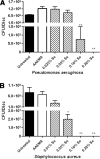
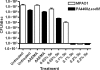
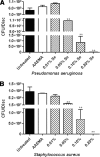
Among the most difficult bacterial infections encountered in treating patients are wound infections, which may occur in burn victims, patients with traumatic wounds, necrotic lesions in people with diabetes, and patients with surgical wounds. Within a wound, infecting bacteria frequently develop biofilms. Many current wound dressings are impregnated with antimicrobial agents, such as silver or antibiotics. Diffusion of the agent(s) from the dressing may damage or destroy nearby healthy tissue as well as compromise the effectiveness of the dressing. In contrast, the antimicrobial agent selenium can be covalently attached to the surfaces of a dressing, prolonging its effectiveness. We examined the effectiveness of an organoselenium coating on cellulose discs in inhibiting Pseudomonas aeruginosa and Staphylococcus aureus biofilm formation. Colony biofilm assays revealed that cellulose discs coated with organoselenium completely inhibited P. aeruginosa and S. aureus biofilm formation. Scanning electron microscopy of the cellulose discs confirmed these results. Additionally, the coating on the cellulose discs was stable and effective after a week of incubation in phosphate-buffered saline. These results demonstrate that 0.2% selenium in a coating on cellulose discs effectively inhibits bacterial attachment and biofilm formation and that, unlike other antimicrobial agents, longer periods of exposure to an aqueous environment do not compromise the effectiveness of the coating.
Figures


References
-
- Araujo, J. C., F. C. Teran, R. A. Oliveira, E. A. Nour, M. A. Montenegro, J. R. Campos, and R. F. Vazoller. 2003. Comparison of hexamethyldisilazane and critical point drying treatments for SEM analysis of anaerobic biofilms and granular sludge. J. Electron Microsc. (Tokyo) 52:429-433. - PubMed
-
- Bayle, C., E. Causse, and F. Couderc. 2004. Determination of aminothiols in body fluids, cells, and tissues by capillary electrophoresis. Electrophoresis 25:1457-1472. - PubMed
-
- Braet, F., R. De Zanger, and E. Wisse. 1997. Drying cells for SEM, AFM and TEM by hexamethyldisilazane: a study on hepatic endothelial cells. J. Microsc. 186:84-87. - PubMed
-
- Cabiscol, E., J. Tamarit, and J. Ros. 2000. Oxidative stress in bacteria and protein damage by reactive oxygen species. Int. Microbiol. 3:3-8. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

