Francisella tularensis directly interacts with the endothelium and recruits neutrophils with a blunted inflammatory phenotype
- PMID: 19346432
- PMCID: PMC2692798
- DOI: 10.1152/ajplung.90332.2008
Francisella tularensis directly interacts with the endothelium and recruits neutrophils with a blunted inflammatory phenotype
Abstract
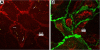
Francisella tularensis, the causative agent of tularemia, is a highly virulent organism, especially when exposure occurs by inhalation. Recent data suggest that Francisella interacts directly with alveolar epithelial cells. Although F. tularensis causes septicemia and can live extracellularly in a murine infection model, there is little information about the role of the vascular endothelium in the host response. We hypothesized that F. tularensis would interact with pulmonary endothelial cells as a prerequisite to the clinically observed recruitment of neutrophils to the lung. Using an in vitro Transwell model system, we studied interactions between F. tularensis live vaccine strain (Ft LVS) and a pulmonary microvascular endothelial cell (PMVEC) monolayer. Organisms invaded the endothelium and were visualized within individual endothelial cells by confocal microscopy. Although these bacteria-endothelial cell interactions did not elicit production of the proinflammatory chemokines, polymorphonuclear leukocytes (PMN) were stimulated to transmigrate across the endothelium in response to Ft LVS. Moreover, transendothelial migration altered the phenotype of recruited PMN; i.e., the capacity of these PMN to activate NADPH oxidase and release elastase in response to subsequent stimulation was reduced compared with PMN that traversed PMVEC in response to Streptococcus pneumoniae. The blunting of PMN responsiveness required PMN transendothelial migration but did not require PMN uptake of Ft LVS, was not dependent on the presence of serum-derived factors, and was not reproduced by Ft LVS-conditioned medium. We speculate that the capacity of Ft LVS-stimulated PMVEC to support transendothelial migration of PMN without triggering release of IL-8 and monocyte chemotactic protein-1 and to suppress the responsiveness of transmigrated PMN to subsequent stimulation could contribute to the dramatic virulence during inhalational challenge with Francisella.
Figures

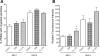
References
-
- Andrews S, Stephens LR, Hawkins PT. PI3K class IB pathway in neutrophils. Sci STKE 2007: CM3, 2007. - PubMed
-
- Boyum A Isolation of mononuclear cells and granulocytes from human blood. J Clin Lab Invest 21: 77–89, 1968. - PubMed
-
- Buckley CD, Ross EA, McGettrick HM, Osborne CE, Haworth O, Schmutz C, Stone PC, Salmon M, Matharu NM, Vohra RK, Nash GB, Rainger GE. Identification of a phenotypically and functionally distinct population of long-lived neutrophils in a model of reverse endothelial migration. J Leukoc Biol 79: 303–311, 2006. - PubMed
-
- Conlan JW, Chen W, Shen H, Webb A, KuoLee R. Experimental tularemia in mice challenged by aerosol or intradermally with virulent strains of Francisella tularensis: bacteriologic and histopathologic studies. Microb Pathog 34: 239–248, 2003. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

