Multilevel analysis of primary metabolism provides new insights into the role of potassium nutrition for glycolysis and nitrogen assimilation in Arabidopsis roots
- PMID: 19346439
- PMCID: PMC2689955
- DOI: 10.1104/pp.108.133629
Multilevel analysis of primary metabolism provides new insights into the role of potassium nutrition for glycolysis and nitrogen assimilation in Arabidopsis roots
Abstract
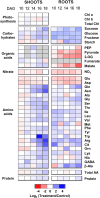
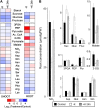
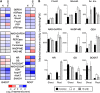
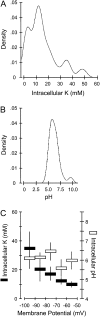
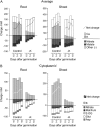
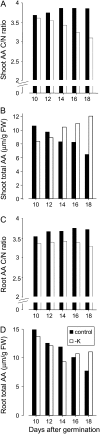
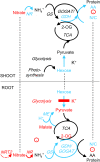
Potassium (K) is required in large quantities by growing crops, but faced with high fertilizer prices, farmers often neglect K application in favor of nitrogen and phosphorus. As a result, large areas of farmland are now depleted of K. K deficiency affects the metabolite content of crops with negative consequences for nutritional quality, mechanical stability, and pathogen/pest resistance. Known functions of K in solute transport, protein synthesis, and enzyme activation point to a close relationship between K and metabolism, but it is unclear which of these are the most critical ones and should be targeted in biotechnological efforts to improve K usage efficiency. To identify metabolic targets and signaling components of K stress, we adopted a multilevel approach combining transcript profiles with enzyme activities and metabolite profiles of Arabidopsis (Arabidopsis thaliana) plants subjected to low K and K resupply. Roots and shoots were analyzed separately. Our results show that regulation of enzymes at the level of transcripts and proteins is likely to play an important role in plant adaptation to K deficiency by (1) maintaining carbon flux into amino acids and proteins, (2) decreasing negative metabolic charge, and (3) increasing the nitrogen-carbon ratio in amino acids. However, changes in transcripts and enzyme activities do not explain the strong and reversible depletion of pyruvate and accumulation of sugars observed in the roots of low-K plants. We propose that the primary cause of metabolic disorders in low-K plants resides in the direct inhibition of pyruvate kinase activity by low cytoplasmic K in root cells.
Figures
References
-
- Amtmann A, Blatt MR (2009) Regulation of macronutrient transport. New Phytol 181 35–52 - PubMed
-
- Amtmann A, Troufflard S, Armengaud P (2008) The effect of potassium nutrition on pest and disease resistance in plants. Physiol Plant 133 682–691 - PubMed
-
- Andrist-Rangel Y, Edwards AC, Hillier S, Oborn I (2007) Long-term K dynamics in organic and conventional mixed cropping systems as related to management and soil properties. Agric Ecosyst Environ 122 413–426
-
- Armengaud P, Zambaux K, Hills A, Sulpice R, Pattison RJ, Blatt MR, Amtmann A (2009) EZ-Rhizo: integrated software for fast and accurate measurement of root system architecture. Plant J 57 945–956 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

