Cells previously identified as retinal stem cells are pigmented ciliary epithelial cells
- PMID: 19346468
- PMCID: PMC2672506
- DOI: 10.1073/pnas.0901596106
Cells previously identified as retinal stem cells are pigmented ciliary epithelial cells
Abstract
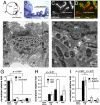
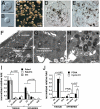
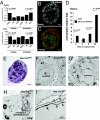
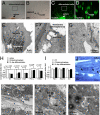
It was previously reported that the ciliary epithelium (CE) of the mammalian eye contains a rare population of cells that could produce clonogenic self-renewing pigmented spheres in culture. Based on their ability to up-regulate genes found in retinal neurons, it was concluded that these sphere-forming cells were retinal stem cells. This conclusion raised the possibility that CE-derived retinal stem cells could help to restore vision in the millions of people worldwide who suffer from blindness associated with retinal degeneration. We report here that human and mouse CE-derived spheres are made up of proliferating pigmented ciliary epithelial cells rather than retinal stem cells. All of the cells in the CE-derived spheres, including the proliferating cells, had molecular, cellular, and morphological features of differentiated pigmented CE cells. These differentiated cells ectopically expressed nestin when exposed to growth factors and low levels of pan-neuronal markers such as beta-III-tubulin. Although the cells aberrantly expressed neuronal markers, they retained their pigmented CE cell morphology and failed to differentiate into retinal neurons in vitro or in vivo. Our results provide an example of a differentiated cell type that can form clonogenic spheres in culture, self-renew, express progenitor cell markers, and initiate neuronal differentiation that is not a stem or progenitor cell. More importantly, our findings highlight the importance of shifting the focus away from studies on CE-derived spheres for cell-based therapies to restore vision in the degenerating retina and improving techniques for using ES cells or retinal precursor cells.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

