Identification of a fluorescent general anesthetic, 1-aminoanthracene
- PMID: 19346473
- PMCID: PMC2672486
- DOI: 10.1073/pnas.0810590106
Identification of a fluorescent general anesthetic, 1-aminoanthracene
Abstract
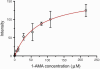
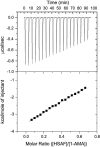
We identified a fluorophore, 1-aminoanthracene (1-AMA), that is anesthetic, potentiates GABAergic transmission, and gives an appropriate dissociation constant, K(d) approximately 0.1 mM, for binding to the general anesthetic site in horse spleen apoferritin (HSAF). 1-AMA fluorescence is enhanced when bound to HSAF. Thus, displacement of 1-AMA from HSAF by other anesthetics attenuates the fluorescence signal and allows determination of K(d), as validated by isothermal titration calorimetry. This provides a unique fluorescence assay for compound screening and anesthetic discovery. Additional electrophysiology experiments in isolated cells indicate that 1-AMA potentiates chloride currents elicited by GABA, similar to many general anesthetics. Furthermore, 1-AMA reversibly immobilizes stage 45-50 Xenopus laevis tadpoles (EC(50) = 16 microM) and fluorescence micrographs show 1-AMA localized to brain and olfactory regions. Thus, 1-AMA provides an unprecedented opportunity for studying general anesthetic distribution in vivo at the cellular and subcellular levels.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

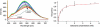




References
-
- Eagle CC, Davis NJ. Report of the anaesthetic mortality committee of Western Australia 1990–1995. Anaesth Intensive Care. 1997;25:51–59. - PubMed
-
- Warden JC, Horan BF. Deaths attributed to anaesthesia in New South Wales, 1984–1990. Anaesth Intensive Care. 1996;24:66–73. - PubMed
-
- Dodds C, Allison J. Postoperative cognitive deficit in the elderly surgical patient. Br J Anaesth. 1998;81:449–462. - PubMed
-
- Moller JT, et al. Long-term postoperative cognitive dysfunction in the elderly: ISPOCD1 study. Lancet. 1998;351:857–861. - PubMed
-
- Parikh SS, Chung F. Postoperative delirium in the elderly. Anesth Analg. 1995;80:1223–1332. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

