Switching direction in electric-signal-induced cell migration by cyclic guanosine monophosphate and phosphatidylinositol signaling
- PMID: 19346484
- PMCID: PMC2672521
- DOI: 10.1073/pnas.0809974106
Switching direction in electric-signal-induced cell migration by cyclic guanosine monophosphate and phosphatidylinositol signaling
Abstract
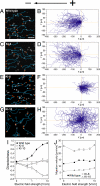
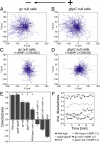
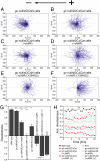
Switching between attractive and repulsive migration in cell movement in response to extracellular guidance cues has been found in various cell types and is an important cellular function for translocation during cellular and developmental processes. Here we show that the preferential direction of migration during electrotaxis in Dictyostelium cells can be reversed by genetically modulating both guanylyl cyclases (GCases) and the cyclic guanosine monophosphate (cGMP)-binding protein C (GbpC) in combination with the inhibition of phosphatidylinositol-3-OH kinases (PI3Ks). The PI3K-dependent pathway is involved in cathode-directed migration under a direct-current electric field. The catalytic domains of soluble GCase (sGC) and GbpC also mediate cathode-directed signaling via cGMP, whereas the N-terminal domain of sGC mediates anode-directed signaling in conjunction with both the inhibition of PI3Ks and cGMP production. These observations provide an identification of the genes required for directional switching in electrotaxis and suggest that a parallel processing of electric signals, in which multiple-signaling pathways act to bias cell movement toward the cathode or anode, is used to determine the direction of migration.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Bray D. Cell Movements: From Molecules to Motility. New York: Garland; 2001.
-
- Song H, et al. Conversion of neuronal growth cone responses from repulsion to attraction by cyclic nucleotides. Science. 1998;281:1515–1518. - PubMed
-
- Nishiyama M, et al. Cyclic AMP/GMP-dependent modulation of Ca2+ channels sets the polarity of nerve growth-cone turning. Nature. 2003;423:990–995. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

