Role of loop-loop interactions in coordinating motions and enzymatic function in triosephosphate isomerase
- PMID: 19348462
- PMCID: PMC2713366
- DOI: 10.1021/bi9002887
Role of loop-loop interactions in coordinating motions and enzymatic function in triosephosphate isomerase
Abstract
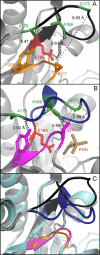
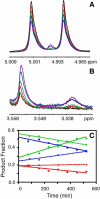
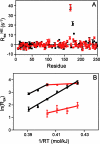
The enzyme triosephosphate isomerase (TIM) has been used as a model system for understanding the relationship between protein sequence, structure, and biological function. The sequence of the active site loop (loop 6) in TIM is directly correlated with a conserved motif in loop 7. Replacement of loop 7 of chicken TIM with the corresponding loop 7 sequence from an archaeal homologue caused a 10(2)-fold loss in enzymatic activity, a decrease in substrate binding affinity, and a decrease in thermal stability. Isotope exchange studies performed by one-dimensional (1)H NMR showed that the substrate-derived proton in the enzyme is more susceptible to solvent exchange for DHAP formation in the loop 7 mutant than for WT TIM. TROSY-Hahn Echo and TROSY-selected R(1rho) experiments indicate that upon mutation of loop 7, the chemical exchange rate for active site loop motion is nearly doubled and that the coordinated motion of loop 6 is reduced relative to that of the WT. Temperature dependent NMR experiments show differing activation energies for the N- and C-terminal hinges in this mutant enzyme. Together, these data suggest that interactions between loop 6 and loop 7 are necessary to provide the proper chemical context for the enzymatic reaction to occur and that the interactions play a significant role in modulating the chemical dynamics near the active site.
Figures


References
-
- Hedstrom L, Szilagyi L, Rutter WJ. Converting trypsin to chymotrypsin: the role of surface loops. Science. 1992;255:1249–1253. - PubMed
-
- Peng T, Zintsmaster JS, Namanja AT, Peng JW. Sequence-specific dynamics modulate recognition specificity in WW domains. Nat Struct Mol Biol. 2007;14:325–331. - PubMed
-
- Venkitakrishnan RP, Zaborowski E, McElheny D, Benkovic SJ, Dyson HJ, Wright PE. Conformational changes in the active site loops of dihydrofolate reductase during the catalytic cycle. Biochemistry. 2004;43:16046–16055. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

