Nuclear accumulation of beta-catenin protein indicates activation of wnt signaling in chemically induced rat nephroblastomas
- PMID: 19348510
- PMCID: PMC2990985
- DOI: 10.2350/08-03-0443.1
Nuclear accumulation of beta-catenin protein indicates activation of wnt signaling in chemically induced rat nephroblastomas
Abstract
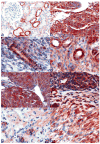
Aberrant wnt signaling caused by mutations in CTNNB1 occurs in about 15% of Wilms tumors, and these mutations appear to be dependent on the concomitant mutational inactivation of the zinc-finger protein WT1. Nuclear beta-catenin protein, a substitute marker of active wnt signaling, has been detected in an even higher proportion (>50%) of Wilms tumors, suggesting alternative genetic pathways leading to beta-catenin activation. Thus, targeting wnt signaling may become an important future therapeutic strategy in Wilms tumor patients. Currently, chemically induced rat nephroblastomas provide the only available rodent model for this tumor. To determine the contribution of active wnt signaling in this model, we investigated 24 chemically induced rat nephroblastomas for beta-catenin protein expression and for Ctnnb1 and WT1 mutations. Immunohistochemistry showed focal strong nuclear accumulation of beta-catenin protein in 18 of 24 tumors, although in a heterogenous pattern. Blastemal and mesenchymal compartments displayed nuclear-positive cells more frequently than areas of epithelial differentiation. Interestingly, we found no mutation of exon 3 of Ctnnb1 and no mutation within the zinc-finger region of WT1 in any of the 24 tumors analyzed. In conclusion, our findings suggest activation of wnt signaling in the majority (63%) of chemically induced rat nephroblastomas. Nuclear expression of beta-catenin in the absence of Ctnnb1 mutations implies, however, alternate mutational targets in rat nephroblastomas.
Figures
Similar articles
-
Myogenesis in Wilms' tumors is associated with mutations of the WT1 gene and activation of Bcl-2 and the Wnt signaling pathway.Pediatr Dev Pathol. 2004 Mar-Apr;7(2):125-37. doi: 10.1007/s10024-003-3023-8. Epub 2004 Mar 4. Pediatr Dev Pathol. 2004. PMID: 14994125
-
Canonical WNT signalling determines lineage specificity in Wilms tumour.Oncogene. 2009 Feb 26;28(8):1063-75. doi: 10.1038/onc.2008.455. Epub 2009 Jan 12. Oncogene. 2009. PMID: 19137020
-
Sequential WT1 and CTNNB1 mutations and alterations of beta-catenin localisation in intralobar nephrogenic rests and associated Wilms tumours: two case studies.J Clin Pathol. 2007 Sep;60(9):1013-6. doi: 10.1136/jcp.2006.043083. Epub 2006 Dec 15. J Clin Pathol. 2007. PMID: 17172473 Free PMC article.
-
The Wnt/beta-catenin pathway in Wilms tumors and prostate cancers.Curr Mol Med. 2007 Aug;7(5):479-89. doi: 10.2174/156652407781387118. Curr Mol Med. 2007. PMID: 17691963 Review.
-
The development of Wilms tumor: from WT1 and microRNA to animal models.Biochim Biophys Acta. 2014 Aug;1846(1):180-7. doi: 10.1016/j.bbcan.2014.07.003. Epub 2014 Jul 11. Biochim Biophys Acta. 2014. PMID: 25018051 Review.
Cited by
-
CITED1 confers stemness to Wilms tumor and enhances tumorigenic responses when enriched in the nucleus.Oncotarget. 2014 Jan 30;5(2):386-402. doi: 10.18632/oncotarget.1566. Oncotarget. 2014. PMID: 24481423 Free PMC article.
-
A comparison of three mucus-secreting airway cell lines (Calu-3, SPOC1 and UNCN3T) for use as biopharmaceutical models of the nose and lung.Eur J Pharm Biopharm. 2021 Oct;167:159-174. doi: 10.1016/j.ejpb.2021.07.016. Epub 2021 Jul 29. Eur J Pharm Biopharm. 2021. PMID: 34332033 Free PMC article.
-
SIX2 and CITED1, markers of nephronic progenitor self-renewal, remain active in primitive elements of Wilms' tumor.J Pediatr Surg. 2012 Jun;47(6):1239-49. doi: 10.1016/j.jpedsurg.2012.03.034. J Pediatr Surg. 2012. PMID: 22703800 Free PMC article.
-
Inhibitory effects of aesculetin on the proliferation of colon cancer cells by the Wnt/β-catenin signaling pathway.Oncol Lett. 2018 May;15(5):7118-7122. doi: 10.3892/ol.2018.8244. Epub 2018 Mar 12. Oncol Lett. 2018. PMID: 29725434 Free PMC article.
-
Wilms' Tumor: A Review of Clinical Characteristics, Treatment Advances, and Research Opportunities.Medicina (Kaunas). 2025 Mar 12;61(3):491. doi: 10.3390/medicina61030491. Medicina (Kaunas). 2025. PMID: 40142302 Free PMC article. Review.
References
-
- Miller RW, Young JL, Jr, Novakovic B. Childhood cancer. Cancer. 1995;75:395–405. - PubMed
-
- Pastore G, Znaor A, Spreafico F, Graf N, Pritchard-Jones K, Steliarova-Foucher E. Malignant renal tumours incidence and survival in European children (1978–1997): report from the Automated Childhood Cancer Information System project. Eur J Cancer. 2006;42:2103–2114. - PubMed
-
- Breslow N, Beckwith JB, Ciol M, Sharples K. Age distribution of Wilms’ tumor: report from the National Wilms’ Tumor Study. Cancer Res. 1988;48:1653–1657. - PubMed
-
- Breslow NE, Langholz B. Childhood cancer incidence: geographical and temporal variations. Int J Cancer. 1983;32:703–716. - PubMed
-
- Matsunaga E. Genetics of Wilms’ tumor. Hum Genet. 1981;57:231–246. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

