The fusion of membranes and vesicles: pathway and energy barriers from dissipative particle dynamics
- PMID: 19348749
- PMCID: PMC2711276
- DOI: 10.1016/j.bpj.2008.11.073
The fusion of membranes and vesicles: pathway and energy barriers from dissipative particle dynamics
Abstract
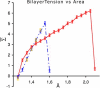
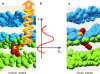
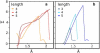
The fusion of lipid bilayers is studied with dissipative particle dynamics simulations. First, to achieve control over membrane properties, the effects of individual simulation parameters are studied and optimized. Then, a large number of fusion events for a vesicle and a planar bilayer are simulated using the optimized parameter set. In the observed fusion pathway, configurations of individual lipids play an important role. Fusion starts with individual lipids assuming a splayed tail configuration with one tail inserted in each membrane. To determine the corresponding energy barrier, we measure the average work for interbilayer flips of a lipid tail, i.e., the average work to displace one lipid tail from one bilayer to the other. This energy barrier is found to depend strongly on a certain dissipative particle dynamics parameter, and, thus, can be adjusted in the simulations. Overall, three subprocesses have been identified in the fusion pathway. Their energy barriers are estimated to lie in the range 8-15 k(B)T. The fusion probability is found to possess a maximum at intermediate tension values. As one decreases the tension, the fusion probability seems to vanish before the tensionless membrane state is attained. This would imply that the tension has to exceed a certain threshold value to induce fusion.
Figures




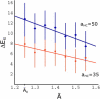

References
-
- Tamm L.K., Crane J., Kiessling V. Membrane fusion: a structural perspective on the interplay of lipids and proteins. Curr. Opin. Struct. Biol. 2003;13:453–466. - PubMed
-
- Lindau M., de Toledo G.A. The fusion pore. Biochim. Biophys. Acta Mol. Cell Res. 2003;1641:167–173. - PubMed
-
- Noguchi H., Takasu M. Fusion pathways of vesicles: a Brownian dynamics simulation. J. Chem. Phys. 2001;115:9547–9551.
-
- Müller M., Katsov K., Schick M. New mechanism of membrane fusion. J. Chem. Phys. 2002;116:2342–2345.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

