Ezrin mediates tethering of the gamma-aminobutyric acid transporter GAT1 to actin filaments via a C-terminal PDZ-interacting domain
- PMID: 19348776
- PMCID: PMC2711277
- DOI: 10.1016/j.bpj.2008.11.070
Ezrin mediates tethering of the gamma-aminobutyric acid transporter GAT1 to actin filaments via a C-terminal PDZ-interacting domain
Abstract
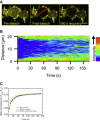
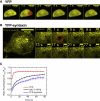
A high density of neurotransmitter transporters on axons and presynaptic boutons is required for the efficient clearance of neurotransmitters from the synapse. Therefore, regulators of transporter trafficking (insertion, retrieval, and confinement) can play an important role in maintaining the transporter density necessary for effective function. We determined the interactions that confine GAT1 at the membrane by investigating the lateral mobility of GAT1-yellow fluorescent protein-8 (YFP8) expressed in neuroblastoma 2a cells. Through fluorescence recovery after photobleaching, we found that a significant fraction ( approximately 50%) of membrane-localized GAT1 is immobile on the time scale investigated ( approximately 150 s). The mobility of the transporter can be increased by depolymerizing actin or by interrupting the GAT1 postsynaptic density 95/Discs large/zona occludens 1 (PDZ)-interacting domain. Microtubule depolymerization, in contrast, does not affect GAT1 membrane mobility. We also identified ezrin as a major GAT1 adaptor to actin. Förster resonance energy transfer suggests that GAT1-YFP8 and cyan fluorescent (CFP) tagged ezrin (ezrin-CFP) exist within a complex that has a Förster resonance energy transfer efficiency of 19% +/- 2%. This interaction can be diminished by disrupting the actin cytoskeleton. In addition, the disruption of actin results in a >3-fold increase in gamma-aminobutyric acid uptake, apparently via a mechanism distinct from the PDZ-interacting protein. Our data reveal that actin confines GAT1 to the plasma membrane via ezrin, and this interaction is mediated through the PDZ-interacting domain of GAT1.
Figures





References
-
- Guastella J., Nelson N., Nelson H., Czyzyk L., Keynan S. Cloning and expression of a rat brain GABA transporter. Science. 1990;249:1303–1306. - PubMed
-
- Morara S., Brecha N.C., Marcotti W., Provini L., Rosina A. Neuronal and glial localization of the GABA transporter GAT-1 in the cerebellar cortex. Neuroreport. 1996;7:2993–2996. - PubMed
-
- Radian R., Kanner B.I. Stoichiometry of sodium- and chloride-coupled γ-aminobutyric acid transport by synaptic plasma membrane vesicles isolated from rat brain. Biochemistry. 1983;22:1236–1241. - PubMed
-
- Kavanaugh M.P., Arriza J.L., North R.A., Amara S.G. Electrogenic uptake of γ-aminobutyric acid by a cloned transporter expressed in Xenopus oocytes. J. Biol. Chem. 1992;267:22007–22009. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

