Chapter 5 Use of ruthenium photooxidation techniques to study electron transfer in the cytochrome bc1 complex
- PMID: 19348884
- PMCID: PMC2761082
- DOI: 10.1016/S0076-6879(08)04405-4
Chapter 5 Use of ruthenium photooxidation techniques to study electron transfer in the cytochrome bc1 complex
Abstract
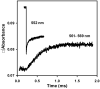
Ruthenium photooxidation methods are presented to study electron transfer between the cytochrome bc(1) complex and cytochrome c and within the cytochrome bc(1) complex. Methods are described to prepare a ruthenium cytochrome c derivative, Ru(z)-39-Cc, by labeling the single sulfhydryl on yeast H39C;C102T iso-1-Cc with the reagent Ru(bpz)(2)(4-bromomethyl-4'-methylbipyridine). The ruthenium complex attached to Cys-39 on the opposite side of Cc from the heme crevice does not affect the interaction with cyt bc(1). Laser excitation of reduced Ru(z)-39-Cc results in photooxidation of heme c within 1 microsec with a yield of 20%. Flash photolysis of a 1:1 complex between reduced yeast cytochrome bc(1) and Ru(z)-39-Cc leads to electron transfer from heme c(1) to heme c with a rate constant of 1.4 x 10(4) s(-1). Methods are described for the use of the ruthenium dimer, Ru(2)D, to photooxidize cyt c(1) in the cytochrome bc(1) complex within 1 microsec with a yield of 20%. Electron transfer from the Rieske iron-sulfur center [2Fe2S] to cyt c(1) was detected with a rate constant of 6 x 10(4) s(-1) in R. sphaeroides cyt bc(1) with this method. This electron transfer step is rate-limited by the rotation of the Rieske iron-sulfur protein in a conformational gating mechanism. This method provides critical information on the dynamics of rotation of the iron-sulfur protein (ISP) as it transfers electrons from QH(2) in the Q(o) site to cyt c(1). These ruthenium photooxidation methods can be used to measure many of the electron transfer reactions in cytochrome bc(1) complexes from any source.
Figures






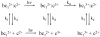
References
-
- Darrouzet E, Daldal F. Movement of the Iron-Sulfur Subunit beyond the ef Loop of Cytochrome b is required for Multiple Turnovers of the bc1 Complex but not for a Single Turnover Qo Site Catalysis. J. Biol. Chem. 2002;277:3471–3476. - PubMed
-
- Durham B, Pan LP, Long J, Millett F. Photoinduced Electron-Transfer Kinetics of Singly Labeled Ruthenium Bis-(bipyridine) Dicarboxybipyridine Cytochrome c Derivatives. Biochemistry. 1989;28:8659–8665. - PubMed
-
- Engstrom G, Xiao K, Yu C-A, Yu L, Durham B, Millett F. Photoinduced electron transfer between the Rieske iron-sulfur protein and cytochrome c(1) in the Rhodobacter sphaeroides cytochrome bc(1) complex. Effects of pH, temperature, and driving force. J. Biol. Chem. 2002;277:31072–31078. - PubMed
-
- Engstrom G, Rajagukguk R, Saunders AJ, Patel CN, Rajagukguk S, Merbitz-Zahradnik T, Xiao K, Pielak GJ, Trumpower B, Yu CA, Yu L, Durham B, Millett F. Design of a ruthenium-labeled cytochrome c derivative to study electron transfer with the cytochrome bc1 complex. Biochemistry. 2003;42:2816–24. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

