Gene expression profiling for nitric oxide prodrug JS-K to kill HL-60 myeloid leukemia cells
- PMID: 19348908
- PMCID: PMC3496159
- DOI: 10.1016/j.ygeno.2009.03.005
Gene expression profiling for nitric oxide prodrug JS-K to kill HL-60 myeloid leukemia cells
Abstract
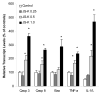
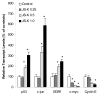
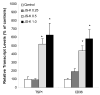
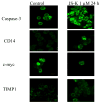
The nitric oxide (NO) prodrug JS-K is shown to have anticancer activity. To profile the molecular events associated with the anticancer effects of JS-K, HL-60 leukemia cells were treated with JS-K and subjected to microarray and real-time RT-PCR analysis. JS-K induced concentration- and time-dependent gene expression changes in HL-60 cells corresponding to the cytolethality effects. The apoptotic genes (caspases, Bax, and TNF-alpha) were induced, and differentiation-related genes (CD14, ITGAM, and VIM) were increased. For acute phase protein genes, some were increased (TP53, JUN) while others were suppressed (c-myc, cyclin E). The expression of anti-angiogenesis genes THBS1 and CD36 and genes involved in tumor cell migration such as tissue inhibitors of metalloproteinases, were also increased by JS-K. Confocal analysis confirmed key gene changes at the protein levels. Thus, multiple molecular events are associated with JS-K effects in killing HL-60, which could be molecular targets for this novel anticancer NO prodrug.
Figures


References
-
- Magrinat G, Mason SN, Shami PJ, Weinberg JB. Nitric oxide modulation of human leukemia cell differentiation and gene expression. Blood. 1992;80:1880–1884. - PubMed
-
- Shami PJ, et al. Nitric oxide modulation of the growth and differentiation of freshly isolated acute non-lymphocytic leukemia cells. Leuk Res. 1995;19:527–533. - PubMed
-
- Shami PJ, Sauls DL, Weinberg JB. Schedule and concentration-dependent induction of apoptosis in leukemia cells by nitric oxide. Leukemia. 1998;12:1461–1466. - PubMed
-
- Keefer LK. Progress toward clinical application of the nitric oxide-releasing diazeniumdiolates. Annu Rev Pharmacol Toxicol. 2003;43:585–607. - PubMed
-
- Saavedra JE, et al. Esterase-sensitive nitric oxide donors of the diazeniumdiolate family: in vitro antileukemic activity. J Med Chem. 2000;43:261–269. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

