Chapter 6: cubic membranes the missing dimension of cell membrane organization
- PMID: 19349040
- PMCID: PMC7105030
- DOI: 10.1016/S1937-6448(08)02006-6
Chapter 6: cubic membranes the missing dimension of cell membrane organization
Abstract
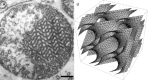
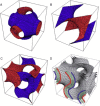
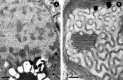
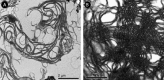
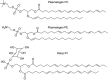
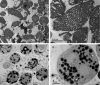
Biological membranes are among the most fascinating assemblies of biomolecules: a bilayer less than 10 nm thick, composed of rather small lipid molecules that are held together simply by noncovalent forces, defines the cell and discriminates between "inside" and "outside", survival, and death. Intracellular compartmentalization-governed by biomembranes as well-is a characteristic feature of eukaryotic cells, which allows them to fulfill multiple and highly specialized anabolic and catabolic functions in strictly controlled environments. Although cellular membranes are generally visualized as flat sheets or closely folded isolated objects, multiple observations also demonstrate that membranes may fold into "unusual", highly organized structures with 2D or 3D periodicity. The obvious correlation of highly convoluted membrane organizations with pathological cellular states, for example, as a consequence of viral infection, deserves close consideration. However, knowledge about formation and function of these highly organized 3D periodic membrane structures is scarce, primarily due to the lack of appropriate techniques for their analysis in vivo. Currently, the only direct way to characterize cellular membrane architecture is by transmission electron microscopy (TEM). However, deciphering the spatial architecture solely based on two-dimensionally projected TEM images is a challenging task and prone to artifacts. In this review, we will provide an update on the current progress in identifying and analyzing 3D membrane architectures in biological systems, with a special focus on membranes with cubic symmetry, and their potential role in physiological and pathophysiological conditions. Proteomics and lipidomics approaches in defined experimental cell systems may prove instrumental to understand formation and function of 3D membrane morphologies.
Figures





References
-
- Adriaensen D., Scheuemann D.W., Timmermans J.-P., de Groodt-Lasseel M.H.A. Neuroepithelial endocrine cells in the lung of the lungfish Protopterus aetiopicus. An electron and fluorescence-microscopical investigation. Acta Anat. 1990;139:70–77. - PubMed
-
- Ahn J.N. Myeloid bodies of the retinal pigment epithelium. I. Distribution, morphology and connections with cytoplasmic organelles. Z. Zellforsch. 1971;115:508–523. - PubMed
-
- Allen R.D. The contractile vacuole and its membrane dynamics. Bioessays. 2000;22:1035–1042. - PubMed
-
- Allen R.D., Fok A.K. Membrane dynamics of the contractile vacuole complex of Paramecium. J. Protozool. 1988;35:63–71.
-
- Allen R.D., Ueno M.S., Pollard L.W., Fok A.K. Monoclonal antibody study of the decorated spongiome of contractile vacuole complexes of Paramecium. J. Cell Sci. 1990;96:469–475. - PubMed
Further reading
-
- Coakley W.T., Gallez D. Membrane-membrane contact: Involvement of interfacial instability in the generation of discrete contacts. Biosci. Rep. 1989;9:675–691. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

