TRAIL, DR4 and DR5 are upregulated in kidneys from patients with lupus nephritis and exert proliferative and proinflammatory effects
- PMID: 19349211
- PMCID: PMC3734543
- DOI: 10.1016/j.clim.2009.02.011
TRAIL, DR4 and DR5 are upregulated in kidneys from patients with lupus nephritis and exert proliferative and proinflammatory effects
Abstract
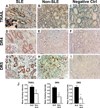
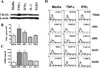
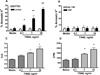
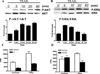
We have previously reported that TRAIL is upregulated on T cells from patients with lupus and that T cell associated TRAIL enhances autoimmune parameters in a murine model of lupus. Whether TRAIL/TRAIL-R interaction plays a role in organ involvement such as lupus nephritis has not yet been assessed. We demonstrate here that TRAIL, DR4 and DR5 are upregulated in proximal and distal tubules of patients with proliferative lupus nephritis. In vitro, expression of TRAIL, DR4 and DR5 on primary proximal tubular epithelial cells (PTEC) was induced by TNFalpha and IFNgamma. Functionally, TRAIL did not induce apoptosis but rather enhanced the proliferation of PTEC through activation of PI3 kinase/AKT and ERK1/2, increased IL-8 production and upregulated ICAM-1 expression. These data demonstrate that cytokine induced upregulation of TRAIL, DR4 and DR5 in tubules from patients with proliferative lupus nephritis may play a protective role by enhancing PTEC survival while also exerting a proinflammatory effect that may contribute to local inflammation and injury.
Figures
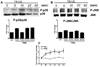
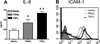
References
-
- Jeremias I, Herr I, Boehler T, Debatin KM. TRAIL/Apo-2-ligand-induced apoptosis in human T cells. Eur.J Immunol. 1998;28:143–152. - PubMed
-
- Morel J, Audo R, Hahne M, Combe B. Tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) induces rheumatoid arthritis synovial fibroblast proliferation through mitogen-activated protein kinases and phosphatidylinositol 3-kinase/Akt. J Biol Chem. 2005;280:15709–15718. - PubMed
-
- Li JH, Kirkiles-Smith NC, McNiff JM, Pober JS. TRAIL induces apoptosis and inflammatory gene expression in human endothelial cells. J Immunol. 2003;171:1526–1533. - PubMed
-
- Wiley SR, Schooley K, Smolak PJ, Din WS, Huang CP, Nicholl JK, Sutherland GR, Smith TD, Rauch C, Smith CA, et al. Identification and characterization of a new member of the TNF family that induces apoptosis. Immunity. 1995;3:673–682. - PubMed
-
- Chaudhary PM, Eby M, Jasmin A, Bookwalter A, Murray J, Hood L. Death receptor 5, a new member of the TNFR family, and DR4 induce FADD-dependent apoptosis and activate the NF-kappaB pathway. Immunity. 1997;7:821–830. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

