Signaling processes for initiating smooth muscle contraction upon neural stimulation
- PMID: 19349274
- PMCID: PMC2708850
- DOI: 10.1074/jbc.M900888200
Signaling processes for initiating smooth muscle contraction upon neural stimulation
Abstract
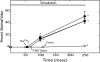
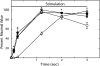
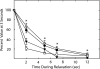
Relationships among biochemical signaling processes involved in Ca2+/calmodulin (CaM)-dependent phosphorylation of smooth muscle myosin regulatory light chain (RLC) by myosin light chain kinase (MLCK) were determined. A genetically-encoded biosensor MLCK for measuring Ca(2+)-dependent CaM binding and activation was expressed in smooth muscles of transgenic mice. We performed real-time evaluations of the relationships among [Ca2+](i), MLCK activation, and contraction in urinary bladder smooth muscle strips neurally stimulated for 3 s. Latencies for the onset of [Ca2+](i) and kinase activation were 55 +/- 8 and 65 +/- 6 ms, respectively. Both increased with RLC phosphorylation at 100 ms, whereas force latency was 109 +/- 3 ms. [Ca2+](i), kinase activation, and RLC phosphorylation responses were maximal by 1.2 s, whereas force increased more slowly to a maximal value at 3 s. A delayed temporal response between RLC phosphorylation and force is probably due to mechanical effects associated with elastic elements in the tissue. MLCK activation partially declined at 3 s of stimulation with no change in [Ca2+](i) and also declined more rapidly than [Ca2+](i) during relaxation. The apparent desensitization of MLCK to Ca2+ activation appears to be due to phosphorylation in its calmodulin binding segment. Phosphorylation of two myosin light chain phosphatase regulatory proteins (MYPT1 and CPI-17) or a protein implicated in strengthening membrane adhesion complexes for force transmission (paxillin) did not change during force development. Thus, neural stimulation leads to rapid increases in [Ca2+](i), MLCK activation, and RLC phosphorylation in phasic smooth muscle, showing a tightly coupled Ca2+ signaling complex as an elementary mechanism initiating contraction.
Figures




References
-
- Kamm K. E., Stull J. T. ( 1985) Annu. Rev. Pharmacol. Toxicol. 25, 593– 620 - PubMed
-
- Tansey M. G., Luby-Phelps K., Kamm K. E., Stull J. T. ( 1994) J. Biol. Chem. 269, 9912– 9920 - PubMed
-
- Zimmermann B., Somlyo A. V., Ellis-Davies G. C., Kaplan J. H., Somlyo A. P. ( 1995) J. Biol. Chem. 270, 23966– 23974 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

