Latent transforming growth factor beta-binding proteins and fibulins compete for fibrillin-1 and exhibit exquisite specificities in binding sites
- PMID: 19349279
- PMCID: PMC2719323
- DOI: 10.1074/jbc.M809348200
Latent transforming growth factor beta-binding proteins and fibulins compete for fibrillin-1 and exhibit exquisite specificities in binding sites
Abstract
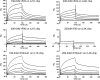
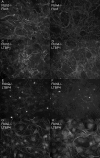
Latent transforming growth factor (TGF) beta-binding proteins (LTBPs) interact with fibrillin-1. This interaction is important for proper sequestration and extracellular control of TGFbeta. Surface plasmon resonance interaction studies show that residues within the first hybrid domain (Hyb1) of fibrillin-1 contribute to interactions with LTBP-1 and LTBP-4. Modulation of binding affinities by fibrillin-1 polypeptides in which residues in the third epidermal growth factor-like domain (EGF3) are mutated demonstrates that the binding sites for LTBP-1 and LTBP-4 are different and suggests that EGF3 may also contribute residues to the binding site for LTBP-4. In addition, fibulin-2, fibulin-4, and fibulin-5 bind to residues contained within EGF3/Hyb1, but mutated polypeptides again indicate differences in their binding sites in fibrillin-1. Results demonstrate that these protein-protein interactions exhibit "exquisite specificities," a phrase commonly used to describe monoclonal antibody interactions. Despite these differences, interactions between LTBP-1 and fibrillin-1 compete for interactions between fibrillin-1 and these fibulins. All of these proteins have been immunolocalized to microfibrils. However, in fibrillin-1 (Fbn1) null fibroblast cultures, LTBP-1 and LTBP-4 are not incorporated into microfibrils. In contrast, in fibulin-2 (Fbln2) null or fibulin-4 (Fbln4) null cultures, fibrillin-1, LTBP-1, and LTBP-4 are incorporated into microfibrils. These data show for the first time that fibrillin-1, but not fibulin-2 or fibulin-4, is required for appropriate matrix assembly of LTBPs. These studies also suggest that the fibulins may affect matrix sequestration of LTBPs, because in vitro interactions between these proteins are competitive.
Figures





References
-
- Charbonneau N. L., Ono R. N., Corson G. M., Keene D. R., Sakai L. Y. ( 2004) Birth Defects Res. C Embryo Today 72, 37– 50 - PubMed
-
- Sengle G., Charbonneau N. L., Ono R. N., Sakai L. Y. ( 2008) Primer on the Metabolic Bone Diseases and Disorders of Mineral Metabolism, 7th Edition, pp. 27– 32, American Society for Bone and Mineral Research, Washington, DC
-
- Charbonneau N. L., Dzamba B. J., Ono R. N., Keene D. R., Corson G. M., Reinhardt D. P., Sakai L. Y. ( 2003) J. Biol. Chem. 278, 2740– 2749 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

