A novel role for tumor necrosis factor-like weak inducer of apoptosis (TWEAK) in the development of cardiac dysfunction and failure
- PMID: 19349318
- PMCID: PMC2924152
- DOI: 10.1161/CIRCULATIONAHA.108.837286
A novel role for tumor necrosis factor-like weak inducer of apoptosis (TWEAK) in the development of cardiac dysfunction and failure
Abstract
Background: Tumor necrosis factor-like weak inducer of apoptosis (TWEAK), a member of the tumor necrosis factor superfamily, is a multifunctional cytokine known to regulate cellular functions in contexts of injury and disease through its receptor, fibroblast growth factor-inducible molecule 14 (Fn14). Although many of the processes and downstream signals regulated by the TWEAK/Fn14 pathway have been implicated in the development of cardiac dysfunction, the role of TWEAK in the cardiovascular system is completely unknown.
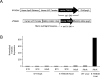
Methods and results: Herein, we demonstrate that mouse and human cardiomyocytes express the TWEAK receptor Fn14. Furthermore, we determine that elevated circulating levels of TWEAK, induced via transgenic or adenoviral-mediated gene expression in mice, result in dilated cardiomyopathy with subsequent severe cardiac dysfunction. This phenotype was mediated exclusively by the Fn14 receptor, independent of tumor necrosis factor-alpha, and was associated with cardiomyocyte elongation and cardiac fibrosis but not cardiomyocyte apoptosis. Moreover, we find that circulating TWEAK levels were differentially upregulated in patients with idiopathic dilated cardiomyopathy compared with other forms of heart disease and normal control subjects.
Conclusions: Our data suggest that TWEAK/Fn14 may be important in regulating myocardial structural remodeling and function and may play a role in the pathogenesis of dilated cardiomyopathy.
Figures













References
-
- Mann DL. Stress-activated cytokines and the heart: from adaptation to maladaptation. Annu Rev Physiol. 2003;65:81–101. - PubMed
-
- Ware CF. The TNF superfamily. Cytokine & growth factor reviews. 2003;14:181–184. - PubMed
-
- Burkly LC, Michaelson JS, Hahm K, Jakubowski A, Zheng TS. TWEAKing tissue remodeling by a multifunctional cytokine: role of TWEAK/Fn14 pathway in health and disease. Cytokine. 2007;40:1–16. - PubMed
-
- Wiley SR, Cassiano L, Lofton T, Davis-Smith T, Winkles JA, Lindner V, Liu H, Daniel TO, Smith CA, Fanslow WC. A novel TNF receptor family member binds TWEAK and is implicated in angiogenesis. Immunity. 2001;15:837–846. - PubMed
-
- Ando T, Ichikawa J, Wako M, Hatsushika K, Watanabe Y, Sakuma M, Tasaka K, Ogawa H, Hamada Y, Yagita H, Nakao A. TWEAK/Fn14 interaction regulates RANTES production, BMP-2-induced differentiation, and RANKL expression in mouse osteoblastic MC3T3-E1 cells. Arthritis research & therapy. 2006;8:R146. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

