Reactive oxygen species mediate liver injury through parenchymal nuclear factor-kappaB inactivation in prolonged ischemia/reperfusion
- PMID: 19349371
- PMCID: PMC2671266
- DOI: 10.2353/ajpath.2009.080857
Reactive oxygen species mediate liver injury through parenchymal nuclear factor-kappaB inactivation in prolonged ischemia/reperfusion
Abstract
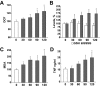
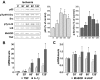
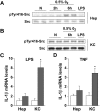
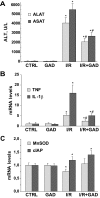
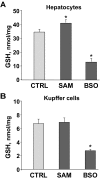
Nuclear factor (NF)-kappaB participates in ischemia/reperfusion (I/R) hepatic signaling, stimulating both protective mechanisms and the generation of inflammatory cytokines. After analyzing NF-kappaB activation during increasing times of ischemia in murine I/R, we observed that the nuclear translocation of p65 paralleled Src and IkappaB tyrosine phosphorylation, which peaked after 60 minutes of ischemia. After extended ischemic periods (90 to 120 minutes) however, nuclear p65 levels were inversely correlated with the progressive induction of oxidative stress. Despite this profile of NF-kappaB activation, inflammatory genes, such as tumor necrosis factor (TNF) and interleukin (IL)-1beta, predominantly induced by Kupffer cells, increased throughout time during ischemia (30 to 120 minutes), whereas protective NF-kappaB-dependent genes, such as manganese superoxide dismutase (Mn-SOD), expressed in parenchymal cells, decreased. Consistent with this behavior, gadolinium chloride pretreatment abolished TNF/IL-1beta up-regulation during ischemia without affecting Mn-SOD levels. Interestingly, specific glutathione (GSH) up-regulation in hepatocytes by S-adenosylmethionine increased Mn-SOD expression and protected against I/R-mediated liver injury despite TNF/IL-1beta induction. Similar protection was achieved by administration of the SOD mimetic MnTBAP. In contrast, indiscriminate hepatic GSH depletion by buthionine-sulfoximine before I/R potentiated oxidative stress and decreased both nuclear p65 and Mn-SOD expression levels, increasing TNF/IL-1beta up-regulation and I/R-induced liver damage. Thus, the divergent role of NF-kappaB activation in selective liver cell populations underlies the dichotomy of NF-kappaB in hepatic I/R injury, illustrating the relevance of specifically maintaining NF-kappaB activation in parenchymal cells.
Figures


References
-
- Luedde T, Trautwein C. Intracellular survival pathways in the liver. Liver Int. 2006;26:1163–1174. - PubMed
-
- Schwabe RF, Brenner DA. Mechanisms of liver injury. I. TNF-alpha-induced liver injury: role of IKK, JNK, and ROS pathways. Am J Physiol. 2006;290:G583–G589. - PubMed
-
- Kuboki S, Okaya T, Schuster R, Blanchard J, Denenberg A, Wong HR, Lentsch AB. Hepatocyte NF-kappaB activation is hepatoprotective during ischemia-reperfusion injury and is augmented by ischemic hypothermia. Am J Physiol. 2007;292:G201–G207. - PubMed
-
- Luedde T, Assmus U, Wüstefeld T, Meyer zu Vilsendorf A, Roskams T, Schmidt-Supprian M, Rajewsky K, Brenner DA, Manns MP, Pasparakis M, Trautwein C. Deletion of IKK2 in hepatocytes does not sensitize these cells to TNF-induced apoptosis but protects from ischemia/reperfusion injury. J Clin Invest. 2005;115:849–859. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

