Adverse antiepileptic drug effects: toward a clinically and neurobiologically relevant taxonomy
- PMID: 19349601
- PMCID: PMC2677485
- DOI: 10.1212/01.wnl.0000345667.45642.61
Adverse antiepileptic drug effects: toward a clinically and neurobiologically relevant taxonomy
Abstract
Background: Adverse effects (AEs) of antiepileptic drugs (AEDs) are a major impediment to optimal dosing for seizure control. Better understanding of clinical properties of AEs is a prerequisite for systematic research of their neurobiological underpinnings. This study aimed to define specific patterns of AE occurrence and determine their clinical relevance based on their association with subjective health status.
Methods: Two hundred subjects with epilepsy completed validated self-report health assessments, including the Adverse Event Profile (AEP) and Quality of Life in Epilepsy Inventory (QOLIE)-89. Factor analysis was performed on the 19 AEP items to identify distinct classes of AEs. Correlations between AE class scores and QOLIE-89 scores were evaluated. Multivariate analysis was used to assess contributions of AE class scores to QOLIE-89 scores after controlling for depression and seizure frequency. Relationships between changes in AE class scores and changes in QOLIE-89 scores were also investigated in a subgroup of 62 subjects enrolled in a randomized trial.
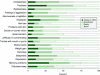
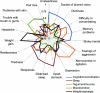
Results: The mean number of AEs per subject was 6.5. AEs were segregated into five classes: Cognition/Coordination, Mood/Emotion, Sleep, Weight/Cephalgia, and Tegument/Mucosa. Higher scores in each AE class were associated with lower QOLIE-89 scores. Cognition/Coordination scores were the strongest predictor of QOLIE-89 scores. Improvements in Cognition/Coordination, Mood/Emotion, and Tegument/Mucosa scores were associated with improvements in QOLIE-89 scores. Improved Cognition/Coordination was the only predictor of improved QOLIE-89.
Conclusion: Adverse effects (AEs) of antiepileptic drugs can be classified in five biologically plausible factors. When specific classes of AEs are identified and attempts are made to reduce them, quality of life is significantly improved.
Figures

Comment in
-
Adverse antiepileptic drug effects.Epilepsy Curr. 2010 Jan;10(1):11-2. doi: 10.1111/j.1535-7511.2009.01339.x. Epilepsy Curr. 2010. PMID: 20126332 Free PMC article. No abstract available.
References
-
- Mattson RH, Cramer JA, Collins JF, et al. Comparison of carbamazepine, phenobarbital, phenytoin, and primidone in partial and secondarily generalized tonic-clonic seizures. N Engl J Med 1985;313:145–151. - PubMed
-
- Cramer JA, Fisher R, Ben-Menachem E, French J, Mattson RH. New antiepileptic drugs: comparison of key clinical trials. Epilepsia 1999;40:590–600. - PubMed
-
- Kwan P, Brodie MJ. Early identification of refractory epilepsy. N Engl J Med 2000;342:314–319. - PubMed
-
- Gilliam F. Optimizing health outcomes in active epilepsy. Neurology 2002;58 (suppl 5):S9–S20. - PubMed
-
- Gilliam FG, Fessler AJ, Baker G, Vahle V, Carter J, Attarian H. Systematic screening allows reduction of adverse antiepileptic drug effects: a randomized trial. Neurology 2004;62:23–27. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
