Effect of varying levels of disease management on smoking cessation: a randomized trial
- PMID: 19349629
- PMCID: PMC2825176
- DOI: 10.7326/0003-4819-150-7-200904070-00003
Effect of varying levels of disease management on smoking cessation: a randomized trial
Abstract
Background: Cigarette smoking is a chronic, relapsing illness that is inadequately addressed in primary care practice.
Objective: To compare cessation rates among smokers who receive pharmacotherapy alone or combined with either moderate- or high-intensity disease management that includes counseling and provider feedback.
Design: Randomized clinical trial from June 2004 to December 2007.
Setting: 50 rural primary care practices.
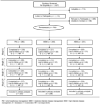
Participants: 750 persons who smoke more than 10 cigarettes per day.
Intervention: Pharmacotherapy alone (n = 250), pharmacotherapy supplemented with up to 2 counseling calls (moderate-intensity disease management) (n = 249), or pharmacotherapy supplemented with up to 6 counseling calls (high-intensity disease management) (n = 251). Interventions were offered every 6 months for 2 years. All participants were offered free pharmacotherapy. Moderate-intensity and high-intensity disease management recipients had postcounseling progress reports faxed to their physicians.
Measurements: Self-reported, point-prevalence smoking abstinence at 24 months (primary outcome) and overall (0 to 24 months) analyses of smoking abstinence, utilization of pharmacotherapy, and discussions about smoking with physicians (secondary outcomes). Research assistants who were blinded to treatment assignment conducted outcome assessments.
Results: Pharmacotherapy utilization was similar across treatment groups, with 473 of 741 (63.8%), 302 of 739 (40.9%), 175 of 732 (23.9%), and 179 of 726 (24.7%) participants requesting pharmacotherapy during the first, second, third, and fourth 6-month treatment cycles, respectively. Of participants who saw a physician during any given treatment cycle, 37.5% to 59.5% reported that they had discussed smoking cessation with their physician; this did not differ across the treatment groups. Abstinence rates increased throughout the study, and overall (0 to 24 months) analyses demonstrated higher abstinence among the high-intensity disease management group than the moderate-intensity disease management group (odds ratio [OR], 1.43 [95% CI, 1.00 to 2.03]) and among the combined disease management groups than the pharmacotherapy-alone group (OR, 1.47 [CI, 1.08 to 2.00]). Self-reported abstinence at 24 months was 68 of 244 (27.9%) and 56 of 238 (23.5%) participants in the high- and moderate-intensity disease management groups, respectively (OR, 1.33 [CI, 0.88 to 2.02]), and 56 of 244 (23.0%) participants in the pharmacotherapy-alone group (OR, 1.12 [CI, 0.78 to 1.61] for combined disease management vs. pharmacotherapy alone).
Limitation: The effect of pharmacotherapy management cannot be separated from the provision of free pharmacotherapy, and cessation was validated in only 58% of self-reported quitters.
Conclusion: Smokers are willing to make repeated pharmacotherapy-assisted quit attempts, leading to progressively greater smoking abstinence. Although point-prevalence abstinence did not differ at 24 months, analyses that incorporated assessments across the full 24 months of treatment suggest that higher-intensity disease management is associated with increased abstinence.
Primary funding source: National Cancer Institute.
Trial registration: ClinicalTrials.gov NCT00440115.
Figures
Comment in
-
The future of tobacco treatment in the health care system.Ann Intern Med. 2009 Apr 7;150(7):496-7. doi: 10.7326/0003-4819-150-7-200904070-00011. Ann Intern Med. 2009. PMID: 19349633 No abstract available.
Summary for patients in
-
Summaries for patients. Comparison of 3 strategies to quit smoking.Ann Intern Med. 2009 Apr 7;150(7):I-36. doi: 10.7326/0003-4819-150-7-200904070-00001. Ann Intern Med. 2009. PMID: 19349626 No abstract available.
References
-
- Fiore MCJC, Baker TB, et al. Treating Tobacco Use and Dependence: 2008 Update. Rockville, MD: US Dept of Health and Human Services; 2008. May [Accessed May 28, 2008]. http://www.ahrq.gov/path/tobacco.htm#Clinic.
-
- Control CfD. Physician and other health-care professional counseling of smokers to quit--United States, 1991. MMWR. 1993;42(44):854–857. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical