Genipin inhibits endothelial exocytosis via nitric oxide in cultured human umbilical vein endothelial cells
- PMID: 19349964
- PMCID: PMC4002818
- DOI: 10.1038/aps.2009.31
Genipin inhibits endothelial exocytosis via nitric oxide in cultured human umbilical vein endothelial cells
Abstract
Aim: Exocytosis of endothelial Weibel-Palade bodies, which contain von Willebrand factor (VWF), P-selectin and other modulators, plays an important role in both inflammation and thrombosis. The present study investigates whether genipin, an aglycon of geniposide, inhibits endothelial exocytosis.
Methods: Human umbilical vein endothelial cells (HUVECs) were isolated from umbilical cords and cultured. The concentration of VWF in cell supernatants was measured using an ELISA Kit. P-selectin translocation on the cell surface was analyzed by cell surface ELISA. Cell viability was measured using a Cell Counting Kit-8. Mouse bleeding times were measured by amputating the tail tip. Western blot analysis was used to determine the amount of endothelial nitric oxide synthase (eNOS) and phospho-eNOS present. Nitric oxide (NO) was measured in the cell supernatants as nitrite using an NO Colorimetric Assay.
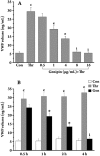
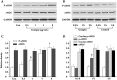
Results: Genipin inhibited thrombin-induced VWF release and P-selectin translocation in HUVECs in a dose- and time-dependent manner. The drug had no cytotoxic effect on the cells at the same doses that were able to inhibit exocytosis. The functional study that demonstrated that genipin inhibited exocytosis in vivo also showed that genipin prolonged the mouse bleeding time. Furthermore, genipin activated eNOS phosphorylation, promoted enzyme activation and increased NO production. L-NAME, an inhibitor of NOS, reversed the inhibitory effects of genipin on endothelial exocytosis.
Conclusion: Genipin inhibits endothelial exocytosis in HUVECs. The mechanism by which this compound inhibits exocytosis may be related to its ability to stimulate eNOS activation and NO production. Our findings suggest a novel anti-inflammatory mechanism for genipin. This compound may represent a new treatment for inflammation and/or thrombosis in which excess endothelial exocytosis plays a pathophysiological role.
Figures





References
-
- Lowenstein CJ, Morrell CN, Yamakuchi M. Regulation of weibel-palade body exocytosis. Trends Cardiovasc Med. 2005;15:302–8. - PubMed
-
- Sugita S. Mechanisms of exocytosis. Acta Physiol (Oxf) 2008;192:185–93. - PubMed
-
- van Mourik JA, Romani de Wit T, Voorberg J. Biogenesis and exocytosis of weibel-palade bodies. Histochem Cell Biol. 2002;117:113–22. - PubMed
-
- Metcalf DJ, Nightingale TD, Zenner HL, Lui-Roberts WW. Cutler DF formation and function of weibel-palade bodies. J Cell Sci. 2008;121:19–27. - PubMed
-
- Geng JG. interaction of vascular endothelial cells with leukocytes, platelets and cancer cells in inflammation, thrombosis and cancer growth and metastasis. Acta Pharmacol Sin. 2003;24:1297–300. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

