Border-cell migration requires integration of spatial and temporal signals by the BTB protein Abrupt
- PMID: 19350016
- PMCID: PMC2675665
- DOI: 10.1038/ncb1863
Border-cell migration requires integration of spatial and temporal signals by the BTB protein Abrupt
Abstract
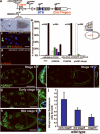
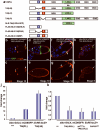
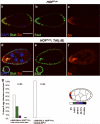
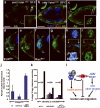
During development, elaborate patterns of cell differentiation and movement must occur in the correct locations and at the proper times. Developmental timing has been studied less than spatial pattern formation, and the mechanisms integrating the two are poorly understood. Border-cell migration in the Drosophila ovary occurs specifically at stage 9. Timing of the migration is regulated by the steroid hormone ecdysone, whereas spatial patterning of the migratory population requires localized activity of the JAK-STAT pathway. Ecdysone signalling is patterned spatially as well as temporally, although the mechanisms are not well understood. In stage 9 egg chambers, ecdysone signalling is highest in anterior follicle cells including the border cells. We identify the gene abrupt as a repressor of ecdysone signalling and border-cell migration. Abrupt protein is normally lost from border-cell nuclei during stage 9, in response to JAK-STAT activity. This contributes to the spatial pattern of the ecdysone response. Abrupt attenuates ecdysone signalling by means of a direct interaction with the basic helix-loop-helix (bHLH) domain of the P160 ecdysone receptor coactivator Taiman (Tai). Taken together, these findings provide a molecular mechanism by which spatial and temporal cues are integrated.
Figures




References
-
- Doe CQ. Chinmo and neuroblast temporal identity. Cell. 2006;127:254–256. - PubMed
-
- Zhu S, et al. Gradients of the Drosophila Chinmo BTB-zinc finger protein govern neuronal temporal identity. Cell. 2006;127:409–422. - PubMed
-
- Moss EG. Heterochronic genes and the nature of developmental time. Curr Biol. 2007;17:R425–434. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

