Stem cell property of postmigratory cranial neural crest cells and their utility in alveolar bone regeneration and tooth development
- PMID: 19350689
- PMCID: PMC2896558
- DOI: 10.1002/stem.2
Stem cell property of postmigratory cranial neural crest cells and their utility in alveolar bone regeneration and tooth development
Abstract
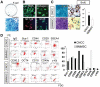
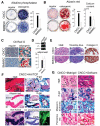
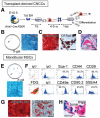
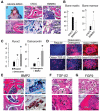
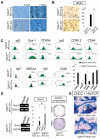
The vertebrate neural crest is a multipotent cell population that gives rise to a variety of different cell types. We have discovered that postmigratory cranial neural crest cells (CNCCs) maintain mesenchymal stem cell characteristics and show potential utility for the regeneration of craniofacial structures. We are able to induce the osteogenic differentiation of postmigratory CNCCs, and this differentiation is regulated by bone morphogenetic protein (BMP) and transforming growth factor-beta signaling pathways. After transplantation into a host animal, postmigratory CNCCs form bone matrix. CNCC-formed bones are distinct from bones regenerated by bone marrow mesenchymal stem cells. In addition, CNCCs support tooth germ survival via BMP signaling in our CNCC-tooth germ cotransplantation system. Thus, we conclude that postmigratory CNCCs preserve stem cell features, contribute to craniofacial bone formation, and play a fundamental role in supporting tooth organ development. These findings reveal a novel function for postmigratory CNCCs in organ development, and demonstrate the utility of these CNCCs in regenerating craniofacial structures.
Figures


References
-
- Noden DM. Cell movements and control of patterned tissue assembly during craniofacial development. J Craniofac Genet Dev Biol. 1991;11:192–213. - PubMed
-
- Le Douarin NM, Ziller C, Coul G. Patterning of neural crest derivatives in the avian embryo: In vivo and in vitro studies. Dev Biol. 1993;159:24–49. - PubMed
-
- Trainor PA, Krumlauf R. Patterning the cranial neural crest: hindbrain segmentation and Hox gene plasticity. Nat Rev Nurosci. 2000;1:116–124. - PubMed
-
- Chai Y, Jiang X, Ito Y, et al. Fate of the mammalian cranial neural crest during tooth and mandibular morphogenesis. Development. 2000;127:1671–1679. - PubMed
-
- Chai Y, Maxson RE., Jr Recent advances in craniofacial morphogenesis. Dev Dyn. 2006;235:2353–2375. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

