Effects of mood on the speed of conscious perception: behavioural and electrophysiological evidence
- PMID: 19351693
- PMCID: PMC2728630
- DOI: 10.1093/scan/nsp010
Effects of mood on the speed of conscious perception: behavioural and electrophysiological evidence
Abstract
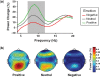
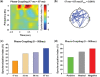
When a visual stimulus is quickly followed in time by a second visual stimulus, we are normally unable to perceive it consciously. This study examined how affective states influence this temporal limit of conscious perception. Using a masked visual perception task, we found that the temporal threshold for access to consciousness is decreased in negative mood and increased in positive mood. To identify the brain mechanisms associated with this effect, we analysed brain oscillations. The mood-induced differences in perception performance were associated with differences in ongoing alpha power (around 10 Hz) before stimulus presentation. Additionally, after stimulus presentation, the better performance during negative mood was associated with enhanced global coordination of neuronal activity of theta oscillations (around 5 Hz). Thus, the effect of mood on the speed of conscious perception seems to depend on changes in oscillatory brain activity, rendering the cognitive system more or less sensitive to incoming stimuli.
Figures

Similar articles
-
The phase of ongoing EEG oscillations uncovers the fine temporal structure of conscious perception.J Neurosci. 2009 Oct 14;29(41):12839-41. doi: 10.1523/JNEUROSCI.3410-09.2009. J Neurosci. 2009. PMID: 19828797 Free PMC article. No abstract available.
-
Occipital EEG correlates of conscious awareness when subjective target shine-through and effective visual masking are compared: bifocal early increase in gamma power and speed-up of P1.Brain Res. 2009 May 19;1271:60-73. doi: 10.1016/j.brainres.2008.12.085. Epub 2009 Mar 25. Brain Res. 2009. PMID: 19328190
-
Early neural correlates of conscious somatosensory perception.J Neurosci. 2005 May 25;25(21):5248-58. doi: 10.1523/JNEUROSCI.0141-05.2005. J Neurosci. 2005. PMID: 15917465 Free PMC article.
-
The speed of visual cognition.Suppl Clin Neurophysiol. 2004;57:617-27. doi: 10.1016/s1567-424x(09)70401-5. Suppl Clin Neurophysiol. 2004. PMID: 16106663 Review.
-
Fluctuations of consciousness, mood, and science: The interhemispheric switch and sticky switch models two decades on.J Comp Neurol. 2020 Dec 1;528(17):3171-3197. doi: 10.1002/cne.24943. Epub 2020 Aug 18. J Comp Neurol. 2020. PMID: 32374025 Review.
Cited by
-
Visual long-term memory is not unitary: Flexible storage of visual information as features or objects as a function of affect.Cogn Affect Behav Neurosci. 2017 Dec;17(6):1141-1150. doi: 10.3758/s13415-017-0538-4. Cogn Affect Behav Neurosci. 2017. PMID: 28924746
-
State-related neural influences on fMRI connectivity estimation.Neuroimage. 2021 Dec 1;244:118590. doi: 10.1016/j.neuroimage.2021.118590. Epub 2021 Sep 21. Neuroimage. 2021. PMID: 34560268 Free PMC article. Review.
-
Differential interference effects of negative emotional states on subsequent semantic and perceptual processing.Emotion. 2011 Dec;11(6):1263-78. doi: 10.1037/a0026329. Emotion. 2011. PMID: 22142207 Free PMC article.
-
CalliFACS: The common marmoset Facial Action Coding System.PLoS One. 2022 May 17;17(5):e0266442. doi: 10.1371/journal.pone.0266442. eCollection 2022. PLoS One. 2022. PMID: 35580128 Free PMC article.
-
Tough doughnuts: affect and the modulation of attention.Front Hum Neurosci. 2013 Dec 18;7:876. doi: 10.3389/fnhum.2013.00876. eCollection 2013. Front Hum Neurosci. 2013. PMID: 24391570 Free PMC article.
References
-
- Anderson A.K. Affective influences on the attentional dynamics supporting awareness. Journal of Experimental Psychology: General. 2005;134:258–81. - PubMed
-
- Anderson A.K., Phelps E.A. Lesions of the human amygdala impair enhanced perception of emotionally salient events. Nature. 2001;411:305–9. - PubMed
-
- Bäuml K.-H., Kuhbandner C. Remembering can cause forgetting—but not in negative moods. Psychological Science. 2007;18:111–5. - PubMed
-
- Bäuml K.-H., Kuhbandner C. Positive moods can eliminate intentional forgetting. Psychonomic Bulletin and Review. 2009;16:93–8. - PubMed
-
- Bless H., Clore G.L., Schwarz N., Golisano V., Rabe C., Wolk M. Mood and the use of scripts: does a happy mood really lead to mindlessness? Journal of Personality and Social Psychology. 1996;71:665–79. - PubMed

