An in-frame deletion in Kir6.2 (KCNJ11) causing neonatal diabetes reveals a site of interaction between Kir6.2 and SUR1
- PMID: 19351728
- PMCID: PMC7611921
- DOI: 10.1210/jc.2009-0159
An in-frame deletion in Kir6.2 (KCNJ11) causing neonatal diabetes reveals a site of interaction between Kir6.2 and SUR1
Abstract
Context: Activating mutations in genes encoding the Kir6.2 (KCNJ11) and SUR1 (ABCC8) subunits of the pancreatic ATP-sensitive K(+) channel are a common cause of permanent neonatal diabetes (PNDM). All Kir6.2 mutations identified to date are missense mutations. We describe here a novel in-frame deletion (residues 28-32) in Kir6.2 in a heterozygous patient with PNDM without neurological problems that are detectable by standard evaluation.
Objective: The aim of the study was to identify the mutation responsible for neonatal diabetes in this patient and characterize its functional effects.
Design: Wild-type and mutant Kir6.2/SUR1 channels were examined by heterologous expression in Xenopus oocytes.
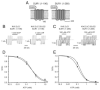
Results: The Kir6.2-28Delta32 mutation produced a significant decrease in ATP inhibition and an increase in whole-cell K(ATP) currents, explaining the diabetes of the patient. Tolbutamide block was only slightly reduced in the simulated heterozygous state, suggesting that the patient should respond to sulfonylurea therapy. The mutation decreased ATP inhibition indirectly, by increasing the intrinsic (unliganded) channel open probability. Neither effect was observed when Kir6.2 was expressed in the absence of SUR1, suggesting that the mutation impairs coupling between SUR1 and Kir6.2. Coimmunoprecipitation studies further revealed that the mutation disrupted a physical interaction between Kir6.2 and residues 1-288 (but not residues 1-196) of SUR1.
Conclusions: We report a novel KCNJ11 mutation causing PNDM. Our results show that residues 28-32 in the N terminus of Kir6.2 interact both physically and functionally with SUR1 and suggest that residues 196-288 of SUR1 are important in this interaction.
Figures





References
-
- Ashcroft FM. The Walter B. Cannon Physiology in Perspective Lecture, 2007.ATP-sensitive K+ channels and disease: from molecule to malady. AmJ Physiol Endocrinol Metab. 2007;293:E880–E889. - PubMed
-
- Gloyn AL, Siddiqui J, Ellard S. Mutations in the genes encoding the pancreatic β-cell KATP channel subunits Kir6.2 (KCNJ11) and SUR1 (ABCC8) in diabetes mellitus and hyperinsulinism. Hum Mutat. 2006;27:220–231. - PubMed
-
- Gloyn AL, Pearson ER, Antcliff JF, Proks P, Bruining GJ, Slingerland AS, Howard N, Srinivasan S, Silva JM, Molnes J, Edghill EL, et al. Activating mutations in the gene encoding the ATP-sensitive potassium-channel subunit Kir6.2 and permanent neonatal diabetes. N Engl J Med. 2004;350:1838–1849. - PubMed
-
- Hattersley AT, Ashcroft FM. Activating mutations in Kir6.2 and neonatal diabetes: new clinical syndromes, new scientific insights, and new therapy. Diabetes. 2005;54:2503–2513. - PubMed

