Human glioblastoma-derived cancer stem cells: establishment of invasive glioma models and treatment with oncolytic herpes simplex virus vectors
- PMID: 19351838
- PMCID: PMC2785462
- DOI: 10.1158/0008-5472.CAN-08-3886
Human glioblastoma-derived cancer stem cells: establishment of invasive glioma models and treatment with oncolytic herpes simplex virus vectors
Abstract
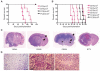
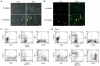
Glioblastoma, the most malignant type of primary brain tumor, is one of the solid cancers where cancer stem cells have been isolated, and studies have suggested resistance of those cells to chemotherapy and radiotherapy. Here, we report the establishment of CSC-enriched cultures derived from human glioblastoma specimens. They grew as neurospheres in serum-free medium with epidermal growth factor and fibroblast growth factor 2, varied in the level of CD133 expression and very efficiently formed highly invasive and/or vascular tumors upon intracerebral implantation into immunodeficient mice. As a novel therapeutic strategy for glioblastoma-derived cancer stem-like cells (GBM-SC), we have tested oncolytic herpes simplex virus (oHSV) vectors. We show that although ICP6 (UL39)-deleted mutants kill GBM-SCs as efficiently as wild-type HSV, the deletion of gamma34.5 significantly attenuated the vectors due to poor replication. However, this was significantly reversed by the additional deletion of alpha47. Infection with oHSV G47Delta (ICP6(-), gamma34.5(-), alpha47(-)) not only killed GBM-SCs but also inhibited their self-renewal as evidenced by the inability of viable cells to form secondary tumor spheres. Importantly, despite the highly invasive nature of the intracerebral tumors generated by GBM-SCs, intratumoral injection of G47Delta significantly prolonged survival. These results for the first time show the efficacy of oHSV against human GBM-SCs, and correlate this cytotoxic property with specific oHSV mutations. This is important for designing new oHSV vectors and clinical trials. Moreover, the new glioma models described in this study provide powerful tools for testing experimental therapeutics and studying invasion and angiogenesis.
Figures



References
-
- Wen PY, Kesari S. Malignant gliomas in adults. N Engl J Med. 2008;359:492–507. - PubMed
-
- Dalerba P, Cho RW, Clarke MF. Cancer stem cells: models and concepts. Annu Rev Med. 2007;58:267–84. - PubMed
-
- Galli R, Binda E, Orfanelli U, et al. Isolation and characterization of tumorigenic, stem-like neural precursors from human glioblastoma. Cancer Res. 2004;64:7011–21. - PubMed
-
- Singh SK, Hawkins C, Clarke ID, et al. Identification of human brain tumour initiating cells. Nature. 2004;432:396–401. - PubMed
-
- Yuan X, Curtin J, Xiong Y, et al. Isolation of cancer stem cells from adult glioblastoma multiforme. Oncogene. 2004;23:9392–400. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

