A point mutation in TRPC3 causes abnormal Purkinje cell development and cerebellar ataxia in moonwalker mice
- PMID: 19351902
- PMCID: PMC2666615
- DOI: 10.1073/pnas.0810599106
A point mutation in TRPC3 causes abnormal Purkinje cell development and cerebellar ataxia in moonwalker mice
Abstract
The hereditary ataxias are a complex group of neurological disorders characterized by the degeneration of the cerebellum and its associated connections. The molecular mechanisms that trigger the loss of Purkinje cells in this group of diseases remain incompletely understood. Here, we report a previously undescribed dominant mouse model of cerebellar ataxia, moonwalker (Mwk), that displays motor and coordination defects and loss of cerebellar Purkinje cells. Mwk mice harbor a gain-of-function mutation (T635A) in the Trpc3 gene encoding the nonselective transient receptor potential cation channel, type C3 (TRPC3), resulting in altered TRPC3 channel gating. TRPC3 is highly expressed in Purkinje cells during the phase of dendritogenesis. Interestingly, growth and differentiation of Purkinje cell dendritic arbors are profoundly impaired in Mwk mice. Our findings define a previously unknown role for TRPC3 in both dendritic development and survival of Purkinje cells, and provide a unique mechanism underlying cerebellar ataxia.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
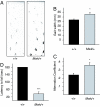
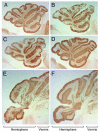
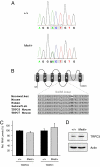
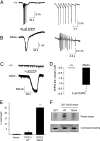
References
-
- Duenas AM, Goold R, Giunti P. Molecular pathogenesis of spinocerebellar ataxias. Brain. 2006;129:1357–1370. - PubMed
-
- Klockgether T. Recent advances in degenerative ataxias. Curr Opin Neurol. 2000;13:451–455. - PubMed
-
- Taroni F, DiDonato S. Pathways to motor incoordination: The inherited ataxias. Nat Rev Neurosci. 2004;5:641–655. - PubMed
-
- Matilla-Duenas A. The highly heterogeneous spinocerebellar ataxias: From genes to targets for therapeutic intervention. Cerebellum. 2008;7:97–100. - PubMed
-
- Lim J, et al. A protein-protein interaction network for human inherited ataxias and disorders of Purkinje cell degeneration. Cell. 2006;125:801–814. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

