Diffuse-type gastric carcinoma: progression, angiogenesis, and transforming growth factor beta signaling
- PMID: 19351925
- PMCID: PMC2669102
- DOI: 10.1093/jnci/djp058
Diffuse-type gastric carcinoma: progression, angiogenesis, and transforming growth factor beta signaling
Abstract
Background: Diffuse-type gastric carcinoma is a cancer with poor prognosis that has high levels of transforming growth factor beta (TGF-beta) expression and thick stromal fibrosis. However, the association of TGF-beta signaling with diffuse-type gastric carcinoma has not been investigated in detail.
Methods: We used a lentiviral infection system to express a dominant-negative TGF-beta type II receptor (dnTbetaRII) or green fluorescent protein (GFP) as a control in the diffuse-type gastric carcinoma cell lines, OCUM-2MLN and OCUM-12. These infected cells and the corresponding parental control cells were subcutaneously or orthotopically injected into nude mice. Angiogenesis was inhibited by infecting cells with a lentivirus carrying the gene for angiogenic inhibitor thrombospondin-1 or by injecting mice intraperitoneally with the small-molecule angiogenic inhibitor sorafenib or with anti-vascular endothelial growth factor (VEGF) neutralizing antibody (six or eight mice per group). Expression of phospho-Smad2 and thrombospondin-1 was investigated immunologically in human gastric carcinoma tissues from 102 patients. All statistical tests were two-sided.
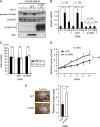
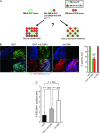
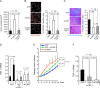
Results: Expression of dnTbetaRII into OCUM-2MLN cells did not affect their proliferation in vitro, but it accelerated the growth of subcutaneously or orthotopically transplanted tumors in vivo (eg, for mean volume of subcutaneous tumors on day 10 relative to that on day 0: dnTbetaRII tumors = 3.49 and GFP tumors = 2.46, difference = 1.02, 95% confidence interval [CI] = 0.21 to 1.84; P = .003). The tumors expressing dnTbetaRII had higher levels of angiogenesis than those expressing GFP because of decreased thrombospondin-1 production. Similar results were obtained with OCUM-12 cells. Expression of thrombospondin-1 in the dnTbetaRII tumor or treatment with sorafenib or anti-VEGF antibody reduced tumor growth, whereas knockdown of thrombospondin-1 expression resulted in more accelerated growth of OCUM-2MLN tumors than of GFP tumors (eg, mean tumor volumes on day 14 relative to those on day 0: thrombospondin-1-knockdown tumors = 4.91 and GFP tumors = 3.79, difference = 1.12, 95% CI = 0.80 to 1.44; P < .001). Positive association between phosphorylated Smad2 and thrombospondin-1 immunostaining was observed in human gastric carcinoma tissues.
Conclusions: Disruption of TGF-beta signaling in diffuse-type gastric carcinoma models appeared to accelerate tumor growth, apparently through increased tumor angiogenesis that was induced by decreased expression of thrombospondin-1.
Figures


References
-
- Hohenberger P, Gretschel S. Gastric cancer. Lancet. 2003;362(9380):305–315. - PubMed
-
- Laurén P. The two histological main types of gastric carcinoma: diffuse and so-called intestinal-type carcinoma. Acta Pathol Microbiol Scand. 1965;64:31–49. - PubMed
-
- Henson DE, Dittus C, Younes M, Nguyen H, Albores-Saavedra J. Differential trends in the intestinal and diffuse types of gastric carcinoma in the United States, 19732000. Arch Pathol Lab Med. 2004;128(7):765–770. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

