Effects of exercise training on health status in patients with chronic heart failure: HF-ACTION randomized controlled trial
- PMID: 19351942
- PMCID: PMC2690699
- DOI: 10.1001/jama.2009.457
Effects of exercise training on health status in patients with chronic heart failure: HF-ACTION randomized controlled trial
Erratum in
- JAMA. 2009 Dec 2;302(21):2322
Abstract
Context: Findings from previous studies of the effects of exercise training on patient-reported health status have been inconsistent.
Objective: To test the effects of exercise training on health status among patients with heart failure.
Design, setting, and patients: Multicenter, randomized controlled trial among 2331 medically stable outpatients with heart failure with left ventricular ejection fraction of 35% or less. Patients were randomized from April 2003 through February 2007.
Interventions: Usual care plus aerobic exercise training (n = 1172), consisting of 36 supervised sessions followed by home-based training, vs usual care alone (n = 1159). Randomization was stratified by heart failure etiology, which was a covariate in all models.
Main outcome measures: Kansas City Cardiomyopathy Questionnaire (KCCQ) overall summary scale and key subscales at baseline, every 3 months for 12 months, and annually thereafter for up to 4 years. The KCCQ is scored from 0 to 100 with higher scores corresponding to better health status. Treatment group effects were estimated using linear mixed models according to the intention-to-treat principle.
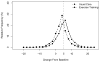
Results: Median follow-up was 2.5 years. At 3 months, usual care plus exercise training led to greater improvement in the KCCQ overall summary score (mean, 5.21; 95% confidence interval, 4.42 to 6.00) compared with usual care alone (3.28; 95% confidence interval, 2.48 to 4.09). The additional 1.93-point increase (95% confidence interval, 0.84 to 3.01) in the exercise training group was statistically significant (P < .001). After 3 months, there were no further significant changes in KCCQ score for either group (P = .85 for the difference between slopes), resulting in a sustained, greater improvement overall for the exercise group (P < .001). Results were similar on the KCCQ subscales, and no subgroup interactions were detected.
Conclusions: Exercise training conferred modest but statistically significant improvements in self-reported health status compared with usual care without training. Improvements occurred early and persisted over time.
Trial registration: clinicaltrials.gov Identifier: NCT00047437.
Figures


References
-
- Dracup K, Walden JA, Stevenson LW, Brecht ML. Quality of life in patients with advanced heart failure. J Heart Lung Transplant. 1992;11(2 Pt 1):273–279. - PubMed
-
- Krumholz HM, Butler J, Miller J, et al. Prognostic importance of emotional support for elderly patients hospitalized with heart failure. Circulation. 1998;97(10):958–964. - PubMed
-
- Metra M, Giubbini R, Nodari S, Boldi E, Modena MG, Dei Cas L. Differential effects of beta-blockers in patients with heart failure: a prospective, randomized, double-blind comparison of the long-term effects of metoprolol versus carvedilol. Circulation. 2000;102(5):546–551. - PubMed
-
- Pires LA, Abraham WT, Young JB, Johnson KM. Clinical predictors and timing of New York Heart Association class improvement with cardiac resynchronization therapy in patients with advanced chronic heart failure: results from the Multicenter InSync Randomized Clinical Evaluation (MIRACLE) and Multicenter InSync ICD Randomized Clinical Evaluation (MIRACLE-ICD) trials. Am Heart J. 2006;151(4):837–843. - PubMed
-
- McKelvie RS. Exercise training in patients with heart failure: clinical outcomes, safety, and indications. Heart Fail Rev. 2008;13(1):3–11. - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
- U01 HL064257/HL/NHLBI NIH HHS/United States
- 5U01HL066461/HL/NHLBI NIH HHS/United States
- 5U01HL064264/HL/NHLBI NIH HHS/United States
- U01 HL064250/HL/NHLBI NIH HHS/United States
- R37 AG018915/AG/NIA NIH HHS/United States
- U01 HL064265/HL/NHLBI NIH HHS/United States
- U01 HL066497/HL/NHLBI NIH HHS/United States
- U01 HL064264/HL/NHLBI NIH HHS/United States
- 5U01HL066482/HL/NHLBI NIH HHS/United States
- 5U01HL066497/HL/NHLBI NIH HHS/United States
- U01 HL068980/HL/NHLBI NIH HHS/United States
- 5U01HL064250/HL/NHLBI NIH HHS/United States
- 5U01HL064265/HL/NHLBI NIH HHS/United States
- U01 HL066491/HL/NHLBI NIH HHS/United States
- 5U01HL068973/HL/NHLBI NIH HHS/United States
- 5U01HL064257/HL/NHLBI NIH HHS/United States
- U01 HL066482/HL/NHLBI NIH HHS/United States
- 5U01HL063747/HL/NHLBI NIH HHS/United States
- 5U01HL066491/HL/NHLBI NIH HHS/United States
- U01 HL066494/HL/NHLBI NIH HHS/United States
- 5U01HL066501/HL/NHLBI NIH HHS/United States
- U01 HL066461/HL/NHLBI NIH HHS/United States
- U01 HL068973/HL/NHLBI NIH HHS/United States
- 5U01HL068980/HL/NHLBI NIH HHS/United States
- U01 HL063747/HL/NHLBI NIH HHS/United States
- 5U01HL066494/HL/NHLBI NIH HHS/United States
- U01 HL066501/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

