The anti-apoptotic activity of BAG3 is restricted by caspases and the proteasome
- PMID: 19352495
- PMCID: PMC2662420
- DOI: 10.1371/journal.pone.0005136
The anti-apoptotic activity of BAG3 is restricted by caspases and the proteasome
Abstract
Background: Caspase-mediated cleavage and proteasomal degradation of ubiquitinated proteins are two independent mechanisms for the regulation of protein stability and cellular function. We previously reported BAG3 overexpression protected ubiquitinated clients, such as AKT, from proteasomal degradation and conferred cytoprotection against heat shock. We hypothesized that the BAG3 protein is regulated by proteolysis.
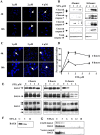
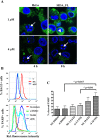
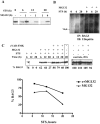
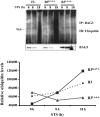
Methodology/principal findings: Staurosporine (STS) was used as a tool to test for caspase involvement in BAG3 degradation. MDA435 and HeLa human cancer cell lines exposed to STS underwent apoptosis with a concomitant time and dose-dependent loss of BAG3, suggesting the survival role of BAG3 was subject to STS regulation. zVAD-fmk or caspase 3 and 9 inhibitors provided a strong but incomplete protection of both cells and BAG3 protein. Two putative caspase cleavage sites were tested: KEVD (BAG3(E345A/D347A)) within the proline-rich center of BAG3 (PXXP) and the C-terminal LEAD site (BAG3(E516A/D518A)). PXXP deletion mutant and BAG3(E345A/D347A), or BAG3(E516A/D518A) respectively slowed or stalled STS-mediated BAG3 loss. BAG3, ubiquitinated under basal growth conditions, underwent augmented ubiquitination upon STS treatment, while there was no increase in ubiquitination of the BAG3(E516A/D518A) caspase-resistant mutant. Caspase and proteasome inhibition resulted in partial and independent protection of BAG3 whereas inhibitors of both blocked BAG3 degradation. STS-induced apoptosis was increased when BAG3 was silenced, and retention of BAG3 was associated with cytoprotection.
Conclusions/significance: BAG3 is tightly controlled by selective degradation during STS exposure. Loss of BAG3 under STS injury required sequential caspase cleavage followed by polyubiquitination and proteasomal degradation. The need for dual regulation of BAG3 in apoptosis suggests a key role for BAG3 in cancer cell resistance to apoptosis.
Conflict of interest statement
Figures


Similar articles
-
BAG3 induces the sequestration of proteasomal clients into cytoplasmic puncta: implications for a proteasome-to-autophagy switch.Autophagy. 2014 Sep;10(9):1603-21. doi: 10.4161/auto.29409. Epub 2014 Jul 10. Autophagy. 2014. PMID: 25046115 Free PMC article.
-
Characterization of BAG3 cleavage during apoptosis of pancreatic cancer cells.J Cell Physiol. 2010 Jul;224(1):94-100. doi: 10.1002/jcp.22097. J Cell Physiol. 2010. PMID: 20232307
-
Caspase-dependent cleavage of BAG3 in proteasome inhibitors-induced apoptosis in thyroid cancer cells.Biochem Biophys Res Commun. 2008 May 9;369(3):894-8. doi: 10.1016/j.bbrc.2008.02.112. Epub 2008 Mar 4. Biochem Biophys Res Commun. 2008. PMID: 18325327
-
HspB8 and Bag3: a new chaperone complex targeting misfolded proteins to macroautophagy.Autophagy. 2008 Feb;4(2):237-9. doi: 10.4161/auto.5407. Epub 2007 Dec 11. Autophagy. 2008. PMID: 18094623 Review.
-
Role of BAG3 in cancer progression: A therapeutic opportunity.Semin Cell Dev Biol. 2018 Jun;78:85-92. doi: 10.1016/j.semcdb.2017.08.049. Epub 2017 Aug 31. Semin Cell Dev Biol. 2018. PMID: 28864347 Review.
Cited by
-
Induction and adaptation of chaperone-assisted selective autophagy CASA in response to resistance exercise in human skeletal muscle.Autophagy. 2015;11(3):538-46. doi: 10.1080/15548627.2015.1017186. Autophagy. 2015. PMID: 25714469 Free PMC article.
-
BAT3 regulates Mycobacterium tuberculosis protein ESAT-6-mediated apoptosis of macrophages.PLoS One. 2012;7(7):e40836. doi: 10.1371/journal.pone.0040836. Epub 2012 Jul 13. PLoS One. 2012. PMID: 22808273 Free PMC article.
-
Identification of a New Promising BAG3 Modulator Featuring the Imidazopyridine Scaffold.Molecules. 2024 Oct 25;29(21):5051. doi: 10.3390/molecules29215051. Molecules. 2024. PMID: 39519692 Free PMC article.
-
Personalization of cancer treatment using predictive simulation.J Transl Med. 2015 Feb 1;13:43. doi: 10.1186/s12967-015-0399-y. J Transl Med. 2015. PMID: 25638213 Free PMC article.
-
Development of a multiparameter flow cytometric assay as a potential biomarker for homologous recombination deficiency in women with high-grade serous ovarian cancer.J Transl Med. 2015 Jul 22;13:239. doi: 10.1186/s12967-015-0604-z. J Transl Med. 2015. PMID: 26198537 Free PMC article.
References
-
- Widmann C, Gibson S, Johnson GL. Caspase-dependent cleavage of signaling proteins during apoptosis. A turn-off mechanism for anti-apoptotic signals. J Biol Chem. 1998;273:7141–7147. - PubMed
-
- Karran L, Dyer MJ. Proteolytic cleavage of molecules involved in cell death or survival pathways: a role in the control of apoptosis? Crit Rev Eukaryot Gene Expr. 2001;11:269–277. - PubMed
-
- Asher G, Reuven N, Shaul Y. 20S proteasomes and protein degradation “by default”. Bioessays. 2006;28:844–849. - PubMed
-
- Takayama S, Xie Z, Reed JC. An evolutionarily conserved family of Hsp70/Hsc70 molecular chaperone regulators. J Biol Chem. 1999;274:781–786. - PubMed
-
- Lee JH, Takahashi T, Yasuhara N, Inazawa J, Kamada S, et al. Bis, a Bcl-2-binding protein that synergizes with Bcl-2 in preventing cell death. Oncogene. 1999;18:6183–6190. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

